“...of all known liquids, water is probably the most studied and least understood.”
Felix Franks, 1972
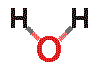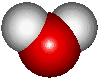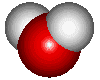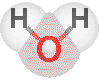
![]() The water molecule
The water molecule
![]() Water: a brief early History of its Science
Water: a brief early History of its Science
![]() The beginnings of water science
The beginnings of water science
![]() The discovery of hydrogen bonds
The discovery of hydrogen bonds
![]() Water
Water
![]() Water's lone pairs
Water's lone pairs
![]() Water electronic structure
Water electronic structure
![]() Water models
Water models
![]() Water reactivity
Water reactivity
![]() Solubility; organic; inorganic
Solubility; organic; inorganic
![]() Easier introduction to the water molecule
Easier introduction to the water molecule
![]() The molecular orbitals of water, H2O
The molecular orbitals of water, H2O
![]() Hydrogen
bonding
Hydrogen
bonding
![]() Introduction
Introduction
![]() Water hydrogen bonds
Water hydrogen bonds
![]() Water hydrogen bond length
Water hydrogen bond length
![]() Water hydrogen bond direction
Water hydrogen bond direction
![]() Hydrogen-bonding and information transfer
Hydrogen-bonding and information transfer
![]() Quantum effects
Quantum effects
![]() Rearranging hydrogen bonds
Rearranging hydrogen bonds
![]() Hydrogen bonding and molecular recognition
Hydrogen bonding and molecular recognition
![]() Bifurcated hydrogen bonds
Bifurcated hydrogen bonds
![]() Information transfer
Information transfer
![]() Hydrogen bonds and solubility
Hydrogen bonds and solubility
![]() Water dimer and small clusters
Water dimer and small clusters
![]() Water dimer and trimer inside fullerenes
Water dimer and trimer inside fullerenes
![]() The water trimer, tetramer, and pentamer
The water trimer, tetramer, and pentamer
![]() The molecular orbitals of a water dimer, (H2O)2
The molecular orbitals of a water dimer, (H2O)2
![]() The molecular orbitals of a water cyclic pentamer, (H2O)5
The molecular orbitals of a water cyclic pentamer, (H2O)5
![]() The phase diagram of water
The phase diagram of water
![]() Triple points
Triple points
![]() The Clausius Clapeyron equation
The Clausius Clapeyron equation
![]() Hexagonally-patterned microdroplets
Hexagonally-patterned microdroplets
![]() Very high-pressure ices including superionic ice
Very high-pressure ices including superionic ice
![]() The 'ice-rules' and ice crystal imperfections
The 'ice-rules' and ice crystal imperfections
![]() Ice crystal data
Ice crystal data
![]() Ice Ic Jmol animation
Ice Ic Jmol animation
![]() Stacking disordered ice (Ice Isd) Jmol animation
Stacking disordered ice (Ice Isd) Jmol animation
![]() Ice II Jmol animation
Ice II Jmol animation
![]() Ice III Jmol animation
Ice III Jmol animation
![]() Ice IV Jmol animation
Ice IV Jmol animation
![]() Ice V Jmol animation
Ice V Jmol animation
![]() Ice VI Jmol animation
Ice VI Jmol animation
![]() Ice VII Jmol animation
Ice VII Jmol animation
![]() Ice VIII Jmol animation
Ice VIII Jmol animation
![]() Ice IX Jmol animation
Ice IX Jmol animation
![]() Ice X Jmol animation
Ice X Jmol animation
![]() Ice XI Jmol animation
Ice XI Jmol animation
![]() Ice XII Jmol animation
Ice XII Jmol animation
![]() Ice XIII Jmol animation
Ice XIII Jmol animation
![]() Ice XIV Jmol animation
Ice XIV Jmol animation
![]() Ice XV Jmol animation
Ice XV Jmol animation
![]() Stacking disordered ice; Ice Isd
Stacking disordered ice; Ice Isd
![]() Ice-sixteen and other ultra low-density ices
Ice-sixteen and other ultra low-density ices
![]() Amorphous ice and glassy water
Amorphous ice and glassy water
![]() Cold metastable glassy water
Cold metastable glassy water
![]() Ultra-viscous water and the glass transition temperature
Ultra-viscous water and the glass transition temperature
![]() Low-density amorphous ice (LDA)
Low-density amorphous ice (LDA)
![]() Medium-density amorphous ice (MDA)
Medium-density amorphous ice (MDA)
![]() High-density amorphous ice (HDA)
High-density amorphous ice (HDA)
![]() Very-high-density amorphous ice (VHDA)
Very-high-density amorphous ice (VHDA)
![]() CS-I clathrate
CS-I clathrate
![]() CS-II clathrate
CS-II clathrate
![]() HS-III clathrate
HS-III clathrate
![]() Other structures
Other structures
![]() Molecular vibration and absorption of water
Molecular vibration and absorption of water
![]() Absorption spectra of gaseous, liquid and solid water
Absorption spectra of gaseous, liquid and solid water
![]() The vibrational spectra of liquid water
The vibrational spectra of liquid water
![]() The visible and UV spectra of liquid water
The visible and UV spectra of liquid water
![]() The spectrum of the Zundel cation
The spectrum of the Zundel cation
![]() Absorption and penetration
Absorption and penetration
![]() Water dissociation, 2H2O = H3O+ + OH−
Water dissociation, 2H2O = H3O+ + OH−
![]() Variation in Kw with temperature and pressure
Variation in Kw with temperature and pressure
![]() Acidity, basicity and the pKa of water
Acidity, basicity and the pKa of water
![]() Ionic strength
Ionic strength
![]() Hydrogen ions
Hydrogen ions
![]() Hydroxide ions
Hydroxide ions
![]() Grotthuss mechanism
Grotthuss mechanism
![]() Diffusion of hydrogen ions
Diffusion of hydrogen ions
![]() Diffusion of hydroxyl ions
Diffusion of hydroxyl ions
![]() The molecular orbitals of the H3O+ and OH− ions
The molecular orbitals of the H3O+ and OH− ions
![]()
![]()
![]() The molecular orbitals of the hydrated hydroxide ion, H3O2−
The molecular orbitals of the hydrated hydroxide ion, H3O2−
![]() The molecular orbitals of the dihydronium ions, H5O2+
The molecular orbitals of the dihydronium ions, H5O2+
![]() Water at interfaces and nanobubbles
Water at interfaces and nanobubbles
![]() Water molecules confined in beryl nanovoids
Water molecules confined in beryl nanovoids
![]() Capillary rise
Capillary rise
![]() Capillary condensation
Capillary condensation
![]() Cavity filling
Cavity filling
![]() Venturi effect
Venturi effect
![]() Capillary flow cause of ring stains
Capillary flow cause of ring stains
![]() Interfacial water and water-gas interfaces
Interfacial water and water-gas interfaces
![]() The surface of ice (Why is ice slippery?)
The surface of ice (Why is ice slippery?)
![]() Metal interfaces
Metal interfaces
![]() Silica interfaces
Silica interfaces
![]() Surface potential
Surface potential
![]() Zeta potential
Zeta potential
![]() Surface charge changes with pH
Surface charge changes with pH
![]() Evaporation and condensation
Evaporation and condensation
![]() Thermodynamics of the liquid-gas surface for water
Thermodynamics of the liquid-gas surface for water
![]() Nanobubbles (ultrafine bubbles)
Nanobubbles (ultrafine bubbles)
![]() Rationale for nanobubble stability
Rationale for nanobubble stability
![]() Nanobubble detection and characterization
Nanobubble detection and characterization
![]() Nanobubbles, preparation, and use
Nanobubbles, preparation, and use
![]() Seventy-four anomalous properties of water
Seventy-four anomalous properties of water
![]() The range of anomalous properties of water
The range of anomalous properties of water
![]() Rationale for the low-temperature anomalies of liquid water
Rationale for the low-temperature anomalies of liquid water
![]() Water has unusually high melting point
Water has unusually high melting point
![]() Water has unusually high boiling point
Water has unusually high boiling point
![]() Water has unusually high critical point
Water has unusually high critical point
![]() Solid water exists in a wide variety of stable structures
Solid water exists in a wide variety of stable structures
![]() The thermal conductivity, shear
modulus and transverse sound velocity of ice reduce with increasing pressure
The thermal conductivity, shear
modulus and transverse sound velocity of ice reduce with increasing pressure
![]() The structure of liquid water changes at high pressure
The structure of liquid water changes at high pressure
![]() Supercooled water has two phases
Supercooled water has two phases
![]() Liquid water is easily supercooled but glassified with difficulty
Liquid water is easily supercooled but glassified with difficulty
![]() A liquid water phase exists at very low temperatures
A liquid water phase exists at very low temperatures
![]() Liquid water may be easily superheated
Liquid water may be easily superheated
![]() Hot water freezes faster than cold water; the Mpemba effect
Hot water freezes faster than cold water; the Mpemba effect
![]() Warm water vibrates longer than cold water
Warm water vibrates longer than cold water
![]() Water molecules shrink as the temperature rises and expand as the pressure increases
Water molecules shrink as the temperature rises and expand as the pressure increases
![]() A liquid-liquid transition occurs at about 330 K.
A liquid-liquid transition occurs at about 330 K.
![]() The density of ice increases on heating (up to 70 K)
The density of ice increases on heating (up to 70 K)
![]() Water expands on freezing
Water expands on freezing
![]() Pressure reduces ice's melting point
Pressure reduces ice's melting point
![]() Cold liquid water has a high-density
that increases on warmining
Cold liquid water has a high-density
that increases on warmining
![]() The surface of water is denser than the bulk
The surface of water is denser than the bulk
![]() Pressure reduces the temperature of maximum density
Pressure reduces the temperature of maximum density
![]() There is a minimum in the density of supercooled water
There is a minimum in the density of supercooled water
![]() Water has a low thermal expansivity
Water has a low thermal expansivity
![]() Water's thermal expansivity reduces at low temperatures
Water's thermal expansivity reduces at low temperatures
![]() Water's thermal expansivity increases with increased pressure
Water's thermal expansivity increases with increased pressure
![]() The number of nearest neighbors increases on melting
The number of nearest neighbors increases on melting
![]() Nearest neighbors increases with temperature
Nearest neighbors increases with temperature
![]() Water has unusually low compressibility
Water has unusually low compressibility
![]() The compressibility drops as temperature increases
The compressibility drops as temperature increases
![]() The compressibility-temperature maximum
The compressibility-temperature maximum
![]() The speed of sound increases with temperature up to 74 °C
The speed of sound increases with temperature up to 74 °C
![]() The speed of sound may show a minimum
The speed of sound may show a minimum
![]() 'Fast sound' is found at high frequencies
'Fast sound' is found at high frequencies
![]() NMR relaxation time is very small at low temperatures
NMR relaxation time is very small at low temperatures
![]() The NMR shift increases to a maximum at low (supercool) temperatures
The NMR shift increases to a maximum at low (supercool) temperatures
![]() The refractive index of water has a maximum value
The refractive index of water has a maximum value
![]() The change in volume as liquid changes to gas is very large
The change in volume as liquid changes to gas is very large
![]() No aqueous solution is ideal
No aqueous solution is ideal
![]() D2O and T2O differ significantly from H2O
D2O and T2O differ significantly from H2O
![]() Liquid H2O and D2O differ significantly in their phase behavior
Liquid H2O and D2O differ significantly in their phase behavior
![]() H2O
and D 2O ices differ significantly in their quantum behavior (nuclear isotope effect)
H2O
and D 2O ices differ significantly in their quantum behavior (nuclear isotope effect)
![]() The mean kinetic energy of water's hydrogen atoms increases at low temperature (disputed)
The mean kinetic energy of water's hydrogen atoms increases at low temperature (disputed)
![]() Solutes have varying effects on water's properties
Solutes have varying effects on water's properties
![]() nonpolar gases solubility decreases with temperature
nonpolar gases solubility decreases with temperature
![]() The dielectric constant of water and ice are high
The dielectric constant of water and ice are high
![]() The relative permittivity shows a temperature maximum
The relative permittivity shows a temperature maximum
![]() The relative permittivity shows a 'kink' in its behavior with temperature at 60 °C
The relative permittivity shows a 'kink' in its behavior with temperature at 60 °C
![]() Proton and hydroxide ion mobilities are anomalously fast
Proton and hydroxide ion mobilities are anomalously fast
![]() The electrical conductivity of water rises to a maximum
The electrical conductivity of water rises to a maximum
![]() The electrical conductivity of water rises with frequency
The electrical conductivity of water rises with frequency
![]() Acidity constants of weak acids show temperature minima
Acidity constants of weak acids show temperature minima
![]() X-ray diffraction shows an unusually detailed structure
X-ray diffraction shows an unusually detailed structure
![]() Under high-pressure, water molecules move apart
Under high-pressure, water molecules move apart
![]() Water adsorption may cause negative electrical resistance
Water adsorption may cause negative electrical resistance
![]() Thermodynamic anomalies T1-T11
Thermodynamic anomalies T1-T11
![]() The heat of fusion of water exhibits a maximum at -17 °C
The heat of fusion of water exhibits a maximum at -17 °C
![]() Water has higher specific heat capacity than ice or steam
Water has higher specific heat capacity than ice or steam
![]() The specific heat capacity (CP and CV) is unusually high
The specific heat capacity (CP and CV) is unusually high
![]() The specific heat capacity CP has a minimum at 36°
The specific heat capacity CP has a minimum at 36°
![]() The specific heat capacity (CP) has a maximum
The specific heat capacity (CP) has a maximum
![]() The specific heat capacity (CP) has a pressure minimum
The specific heat capacity (CP) has a pressure minimum
![]() The heat capacity (CV) has a maximum
The heat capacity (CV) has a maximum
![]() High heat of vaporization
High heat of vaporization
![]() High heat of sublimation
High heat of sublimation
![]() High entropy of vaporization
High entropy of vaporization
![]() The thermal conductivity of water is high
The thermal conductivity of water is high
![]() Water has unusually high viscosity
Water has unusually high viscosity
![]() Large viscosity and Prandtl number increase as the temperature is lowered
Large viscosity and Prandtl number increase as the temperature is lowered
![]() Water's viscosity decreases with pressure below 33 °C
Water's viscosity decreases with pressure below 33 °C
![]() Large diffusion decrease as the temperature is lowered
Large diffusion decrease as the temperature is lowered
![]() The self-diffusion of water increases with the density
The self-diffusion of water increases with the density
![]() The thermal diffusivity rises to a maximum at about 0.8 GPa
The thermal diffusivity rises to a maximum at about 0.8 GPa
![]() Water has unusually high surface tension
Water has unusually high surface tension
![]() Some salts give a surface tension minimum; the Jones-Ray effect
Some salts give a surface tension minimum; the Jones-Ray effect
![]() Some salts prevent the coalescence of small bubbles
Some salts prevent the coalescence of small bubbles
![]() The molar ionic volumes of salts show maxima with respect to temperature
The molar ionic volumes of salts show maxima with respect to temperature
![]() Unexpected properties of water
Unexpected properties of water
![]() Vapor pressure-Temperature behavior
Vapor pressure-Temperature behavior
![]() Pressure-Temperature-Density behavior
Pressure-Temperature-Density behavior
![]() Temperature-viscosity behavior
Temperature-viscosity behavior
![]() Temperature-enthalpy of vaporization relationship
Temperature-enthalpy of vaporization relationship
![]() Logarithmic relationships emanating from 228 K
Logarithmic relationships emanating from 228 K
![]() Properties of water and its isotopologues
Properties of water and its isotopologues
![]() Vienna Standard Mean Ocean Water
Vienna Standard Mean Ocean Water
![]() Short properties list for liquid H2O
Short properties list for liquid H2O
![]() Changes in some properties
with temperature (-30 °C - 100 °C)
Changes in some properties
with temperature (-30 °C - 100 °C)
![]() Changes in some further properties
with temperature (0 °C - 373 °C)
Changes in some further properties
with temperature (0 °C - 373 °C)
![]() Water-related molecules; comparative data
Water-related molecules; comparative data
![]() The temperature scale and absolute zero>
The temperature scale and absolute zero>
![]() The Zeroth Law of Thermodynamics
The Zeroth Law of Thermodynamics
![]() The First Law of Thermodynamics
The First Law of Thermodynamics
![]() The Second Law of Thermodynamics
The Second Law of Thermodynamics
![]() The Third Law of Thermodynamics
The Third Law of Thermodynamics
![]() Chemical potential
Chemical potential
![]() Internal energy
Internal energy
![]() Heat capacity and specific heat
Heat capacity and specific heat
![]() Important constants and conversion factors
Important constants and conversion factors
![]() Water model descriptions
Water model descriptions
![]() Water model properties
Water model properties
![]() The Lennard-Jones relationship
The Lennard-Jones relationship
![]() Water clustering in liquid water
Water clustering in liquid water
![]() Overview of the structuring in liquid water
Overview of the structuring in liquid water
![]() Water clustering
Water clustering
![]() Cluster lifetimes and hydrogen-bond lifetimes are independent
Cluster lifetimes and hydrogen-bond lifetimes are independent
![]() Icosahedral water cluster
Icosahedral water cluster
![]() The lifetime of the clusters
The lifetime of the clusters
![]() Introduction to water clustering
Introduction to water clustering
![]() Water's two-state cluster history
Water's two-state cluster history
![]() Is liquid water one liquid or two?
Is liquid water one liquid or two?
![]() Icosahedral clustering and the two-state
mixture model?
Icosahedral clustering and the two-state
mixture model?
![]() Outline of methods for investigating water structure
Outline of methods for investigating water structure
![]() Why different methods give different water structures?
Why different methods give different water structures?
![]() Dielectric spectroscopy
Dielectric spectroscopy
![]() Diffraction methods
Diffraction methods
![]() Modeling
Modeling
![]() Nuclear Magnetic Resonance (NMR)
Nuclear Magnetic Resonance (NMR)
![]() Osmotic stress
Osmotic stress
![]() Physical properties
Physical properties
![]() Vibrational spectra (Raman and infrared)
Vibrational spectra (Raman and infrared)
![]() Terahertz (THz) absorption spectroscopy
Terahertz (THz) absorption spectroscopy
![]() Microwave (GHz) absorption spectroscopy
Microwave (GHz) absorption spectroscopy
![]() Sum frequency generation (SFG)
Sum frequency generation (SFG)
![]() X-Ray spectroscopy
X-Ray spectroscopy
![]() Gaussian and Lorentzian curves
Gaussian and Lorentzian curves
![]() (H2O)100 and (H2O)280 clusters Jmol animation
(H2O)100 and (H2O)280 clusters Jmol animation
![]() (H2O)280 cluster equilibria Jmol animation
(H2O)280 cluster equilibria Jmol animation
![]() Tetrahedral (H2O)14 cluster Jmol animation
Tetrahedral (H2O)14 cluster Jmol animation
![]() The icosahedral (H2O)280 water clusters
The icosahedral (H2O)280 water clusters
![]() Tetrahedral units
Tetrahedral units
![]() Icosahedral clusters
Icosahedral clusters
![]() Cluster equilibria
Cluster equilibria
![]() Cluster equilibria details
Cluster equilibria details
![]() Cluster density
Cluster density
![]() Sub-structures of the icosahedral water cluster
Sub-structures of the icosahedral water cluster
![]() Connectivity map of the water icosahedron
Connectivity map of the water icosahedron
![]() Solid geometry of the icosahedral cluster (Java)
Solid geometry of the icosahedral cluster (Java)
![]() Super-cluster ((H2O)280)13 Jmol animation
Super-cluster ((H2O)280)13 Jmol animation
![]() Super network ((H2O)100)n Jmol animation
Super network ((H2O)100)n Jmol animation
![]() Cluster puckering Jmol animation
Cluster puckering Jmol animation
![]() Tetrahedra cluster Jmol animation
Tetrahedra cluster Jmol animation
![]() Bicyclo-octamers cluster Jmol animation
Bicyclo-octamers cluster Jmol animation
![]() Super-clusters of water molecules
Super-clusters of water molecules
![]() Water cluster equilibria, puckering and temperature effects
Water cluster equilibria, puckering and temperature effects
![]()
![]() Water icosahedral cluster architecture
Water icosahedral cluster architecture
![]() Spherical coordinates of the icosahedral water clusters
Spherical coordinates of the icosahedral water clusters
![]() Shell radii and occupancy of the icosahedral water clusters
Shell radii and occupancy of the icosahedral water clusters
![]() Alternative icosahedral clusters Jmol animation
Alternative icosahedral clusters Jmol animation
![]() Alternative tetrahedral clusters Jmol animation
Alternative tetrahedral clusters Jmol animation
![]() Cavities and networks, Jmol animation
Cavities and networks, Jmol animation
![]() Clathrate-like, Jmol animation
Clathrate-like, Jmol animation
![]() Super-clusters of water molecules
Super-clusters of water molecules
![]() Alternative icosahedral clustering of water
Alternative icosahedral clustering of water
![]() Alternative tetrahedral clustering of water
Alternative tetrahedral clustering of water
![]() Water cluster architecture, based on gas clathrates
Water cluster architecture, based on gas clathrates
![]() Paper model of an icosahedral water structure
Paper model of an icosahedral water structure
![]() Paper model of a truncated icosahedral water structure
Paper model of a truncated icosahedral water structure
![]() Plain paper model of the layers of a truncated icosahedral water structure
Plain paper model of the layers of a truncated icosahedral water structure
![]() Evidence
for icosahedral water clusters
Evidence
for icosahedral water clusters
![]() The radial distribution function
The radial distribution function
![]() Other support from diffraction data
Other support from diffraction data
![]() How
can a liquid have a structure?
How
can a liquid have a structure?
![]() Does
the radial distribution peak at about 3.7
Å exist?
Does
the radial distribution peak at about 3.7
Å exist?
![]() Is
there a fine structure in the radial distribution function?
Is
there a fine structure in the radial distribution function?
![]() Do
interstitial water molecules exist?
Do
interstitial water molecules exist?
![]() Support from clathrate structures
Support from clathrate structures
![]() Evidence from amorphous ice and low-density water
Evidence from amorphous ice and low-density water
![]() Other evidence
Other evidence
![]() The fragile to strong transition
The fragile to strong transition
![]() Hydration
Hydration
![]() The contribution of water to protein structure
The contribution of water to protein structure
![]() Water in protein recognition and binding
Water in protein recognition and binding
![]() Water in protein and enzyme function
Water in protein and enzyme function
![]() Enzymic reactions in biphasic liquid systems
Enzymic reactions in biphasic liquid systems
![]() Protein
folding and denaturation
Protein
folding and denaturation
![]() Protein folding
Protein folding
![]() Protein crystallization
Protein crystallization
![]() Protein denaturation
Protein denaturation
![]() Prions
Prions
![]() Hydrogen bonds in the nucleic acids
Hydrogen bonds in the nucleic acids
![]() Cholesterol and membrane hydration
Cholesterol and membrane hydration
![]() Aqueous properties of the cyclodextrins
Aqueous properties of the cyclodextrins
![]() Polysaccharide
hydration
Polysaccharide
hydration
![]() Hydration
Hydration
![]() Alternatives for defining bound and unbound water
Alternatives for defining bound and unbound water
![]() Polar effects, for example, α-D-galacturonic acid
Polar effects, for example, α-D-galacturonic acid
![]() Weak hydrogen-bonding, for example, α-L-arabinofuranose
Weak hydrogen-bonding, for example, α-L-arabinofuranose
![]() Strong hydrogen-bonding, for example, β-1-4-linked D-xylose
Strong hydrogen-bonding, for example, β-1-4-linked D-xylose
![]() Hydrophobic effects, for example, β-1-4-linked D-xylose
Hydrophobic effects, for example, β-1-4-linked D-xylose
![]() Effects of other solutes: non-ionic
Effects of other solutes: non-ionic
![]() Effects of other solutes: ionic
Effects of other solutes: ionic
![]() Conclusions concerning polysaccharide hydration
Conclusions concerning polysaccharide hydration
![]() Introduction
to polysaccharides
Introduction
to polysaccharides
![]() Chart showing the furan pseudo-rotational
angles of ribose and deoxyribose
Chart showing the furan pseudo-rotational
angles of ribose and deoxyribose
![]() Mixtures of hydrocolloids
Mixtures of hydrocolloids
![]() Effect on viscosity
Effect on viscosity
![]() Hydrocolloid action
Hydrocolloid action
![]() Hydrogels
Hydrogels
![]() Agar Jmol animation
Agar Jmol animation
![]() Alginate Jmol animation
Alginate Jmol animation
![]() Arabinoxylan Jmol animation
Arabinoxylan Jmol animation
![]() Carrageenan Jmol animation
Carrageenan Jmol animation
![]() CMC Jmol animation
CMC Jmol animation
![]() Cellulose Jmol animation
Cellulose Jmol animation
![]() Curdlan Jmol animation
Curdlan Jmol animation
![]() Gelatin Jmol animation
Gelatin Jmol animation
![]() Source
Source
![]() Structural unit
Structural unit
![]() Molecular structure
Molecular structure
![]() Functionality
Functionality
![]() Source
Source
![]() Structural unit
Structural unit
![]() Molecular structure
Molecular structure
![]() Functionality
Functionality
![]() Source
Source
![]() Structural unit
Structural unit
![]() Molecular structure
Molecular structure
![]() Functionality
Functionality
![]() Source
Source
![]() Structural unit
Structural unit
![]() Molecular structure, κ-, ι-, λ- carrageenans,
Helices
Molecular structure, κ-, ι-, λ- carrageenans,
Helices
![]() Functionality
Functionality
![]() Source
Source
![]() Structural unit
Structural unit
![]() Molecular structure
Molecular structure
![]() Functionality
Functionality
![]() Sources for cellulose
Sources for cellulose
![]() Structural unit
Structural unit
![]() Molecular structure
Molecular structure
![]() Microcrystalline cellulose
Microcrystalline cellulose
![]() Functionality
Functionality
![]() Source
Source
![]() Structural unit
Structural unit
![]() Molecular structure
Molecular structure
![]() Functionality
Functionality
![]() Sources for gelatin
Sources for gelatin
![]() Structural unit
Structural unit
![]() Molecular structure
Molecular structure
![]() Functionality and concerns
Functionality and concerns
![]() Source
Source
![]() Structural unit
Structural unit
![]() Molecular structure
Molecular structure
![]() Functionality
Functionality
![]() Source
Source
![]() Structural unit
Structural unit
![]() Molecular structure
Molecular structure
![]() Functionality
Functionality
![]() Source
Source
![]() Structural unit
Structural unit
![]() Molecular structure
Molecular structure
![]() Functionality
Functionality
![]() Other similar gums
Other similar gums
![]() Guar gum Jmol animation
Guar gum Jmol animation
![]() Locust bean gum Jmol animation
Locust bean gum Jmol animation
![]() Pectin Jmol animation
Pectin Jmol animation
![]() Starch Jmol animation
Starch Jmol animation
![]() Xanthan gum Jmol animation
Xanthan gum Jmol animation
![]() Source
Source
![]() Structural unit
Structural unit
![]() Molecular structure
Molecular structure
![]() Functionality
Functionality
![]() Source
Source
![]() Structural unit
Structural unit
![]() Molecular structure
Molecular structure
![]() Functionality
Functionality
![]() Pectin sources
Pectin sources
![]() Pectin structural unit
Pectin structural unit
![]() Molecular structure
Molecular structure
![]() Functionality
Functionality
![]() Sources for starch
Sources for starch
![]() Structural unit
Structural unit
![]() Molecular structure
Molecular structure
![]() Functionality
Functionality
![]() Source
Source
![]() Structural unit
Structural unit
![]() Molecular structure
Molecular structure
![]() Functionality
Functionality
![]() Viscosity
Viscosity
![]() Viscoelasticity
Viscoelasticity
![]() Structural effects
Structural effects
![]() Further rheological terminology
Further rheological terminology
![]() Hydrocolloids
and health (Dietary fiber)
Hydrocolloids
and health (Dietary fiber)
![]() The effect of dietary fiber in digestion
The effect of dietary fiber in digestion
![]() The colon
The colon
![]() Colonic fermentation
Colonic fermentation
![]() Water-holding capacity (WHC)
Water-holding capacity (WHC)
![]() Viscosity and gel formation
Viscosity and gel formation
![]() Binding to bile acids
Binding to bile acids
![]() Ion hydration and aqueous solutions of salts
Ion hydration and aqueous solutions of salts
![]() What is meant by ion hydration
What is meant by ion hydration
![]() Methods for determining ion hydration
Methods for determining ion hydration
![]() Ion pairs
Ion pairs
![]() Water clustering around ions
Water clustering around ions
![]() Sulfate and other large anions
Sulfate and other large anions
![]() The H3O+ magic number cluster ions
The H3O+ magic number cluster ions
![]() Water clustering around the SO42− cluster
Water clustering around the SO42− cluster
![]() Carbon dioxide hydration and equilibria
Carbon dioxide hydration and equilibria
![]() Carbon dioxide compared with carbon monoxide
Carbon dioxide compared with carbon monoxide
![]() Nitrogen oxides | N2O | NO | NO2
Nitrogen oxides | N2O | NO | NO2
![]() Nitrous and nitric acid
Nitrous and nitric acid
![]() Why is hydrofluoric acid a weak acid?
Why is hydrofluoric acid a weak acid?
![]() Orthosilicic acid
Orthosilicic acid
![]() Silica interfaces
Silica interfaces
![]() Rationale
Rationale
![]() Thermodynamic properties
Thermodynamic properties
![]() Effect on physical properties
Effect on physical properties
![]() Effect on solubility; ion-pairs
Effect on solubility; ion-pairs
![]() Stabilization of proteins
Stabilization of proteins
![]() Hydrophobic and hydrophilic associations
Hydrophobic and hydrophilic associations
![]() The effect on biphasic partitioning
The effect on biphasic partitioning
![]() Definitions of kosmotropes
and chaotropes
Definitions of kosmotropes
and chaotropes
![]() Ionic kosmotropes and chaotropes
Ionic kosmotropes and chaotropes
![]() Non-ionic kosmotropes and chaotropes
Non-ionic kosmotropes and chaotropes
![]() Urea
Urea
![]() Imidazole
Imidazole
![]() Guanidinium
Guanidinium
![]() α,α-Trehalose
α,α-Trehalose
![]() Ectoine
Ectoine
![]() Trimethylamine N-oxide (TMAO)
Trimethylamine N-oxide (TMAO)
![]() Hyaluronic acid
Hyaluronic acid
![]() Hydrophobic versus hydrophilic interfaces
Hydrophobic versus hydrophilic interfaces
![]() Extensive hydrophobic interfaces
Extensive hydrophobic interfaces
![]() Solubility effects
Solubility effects
![]() Hydrotropes
Hydrotropes
![]() Salting-out and salting-in
Salting-out and salting-in
![]() Young's equation
Young's equation
![]() Alcoholic solutions
Alcoholic solutions
![]() Water transfer across membranes
Water transfer across membranes
![]() Intracellular water
Intracellular water
![]() Intracellular solutions contain more K+ ions
Intracellular solutions contain more K+ ions
![]() Membranes help create a tendency towards low-density water in cells
Membranes help create a tendency towards low-density water in cells
![]() The effect of intracellular protein on water structuring
The effect of intracellular protein on water structuring
![]() The importance of protein carboxylate groups
The importance of protein carboxylate groups
![]() The importance of protein mobility
The importance of protein mobility
![]() Cooperative conversion of the water structuring
Cooperative conversion of the water structuring
![]() Actin, tubulins and the intermediate filaments
Actin, tubulins and the intermediate filaments
![]() How many planets have life in the Universe
How many planets have life in the Universe
![]() Water on Earth
Water on Earth
![]() Water in the mantle
Water in the mantle
![]() Seawater
Seawater
![]() Water and global warming
Water and global warming
![]() Calcium carbonate equilibria
Calcium carbonate equilibria
![]() Water softening
Water softening
![]() Magnetic descaling
Magnetic descaling
![]() Solar powered water harvesting
Solar powered water harvesting
![]() Treatment of contaminated water
Treatment of contaminated water
![]() Can
life exist without water?
Can
life exist without water?
![]() Consequences of changes in water’s hydrogen bond strength
Consequences of changes in water’s hydrogen bond strength
![]() Estimating the effect of changes in water hydrogen bond strength
Estimating the effect of changes in water hydrogen bond strength
![]() Effect of water hydrogen bond strength on melting and boiling point
Effect of water hydrogen bond strength on melting and boiling point
![]() Effect of hydrogen bond strength on the temperature of maximum density
Effect of hydrogen bond strength on the temperature of maximum density
![]() Effect of water hydrogen bond strength on kosmotropes and chaotropes
Effect of water hydrogen bond strength on kosmotropes and chaotropes
![]() Effect of water hydrogen bond strength on its dissociation
Effect of water hydrogen bond strength on its dissociation
![]() Effect of water hydrogen bond strength on biomolecule hydration
Effect of water hydrogen bond strength on biomolecule hydration
![]() Effect of water hydrogen bond strength on its other physical properties
Effect of water hydrogen bond strength on its other physical properties
![]() Conclusions concerning water and life
Conclusions concerning water and life
![]() Water content
Water content
![]() Water balance
Water balance
![]() Water requirements
Water requirements
![]() Water roles
Water roles
![]() Hydration
Hydration
![]() Drinking water
Drinking water
![]() Water redox processes
Water redox processes
![]() The redox potential of water
The redox potential of water
![]() Magnetic
and electric effects on water
Magnetic
and electric effects on water
![]() Electric effects on water
Electric effects on water
![]() Magnetic effects on water
Magnetic effects on water
![]() Electromagnetic effects on water
Electromagnetic effects on water
![]() Magnetic descaling
Magnetic descaling
![]() Other related effects
Other related effects
![]() Dielectric loss
Dielectric loss
![]() Effect of salt
Effect of salt
![]() Electromagnetic penetration
Electromagnetic penetration
![]() Dielectric constant and polarization
Dielectric constant and polarization
![]() Background information and definitions
Background information and definitions
![]() Temperature and pressure
Temperature and pressure
![]() Polarization and polarizability
Polarization and polarizability
![]() Refractive index
Refractive index
![]() The complex dielectric
permittivity behavior of water
The complex dielectric
permittivity behavior of water
![]() The complex dielectric permittivity
The complex dielectric permittivity
![]() Polarization
Polarization
![]() Reaction of water with ionizing radiation
Reaction of water with ionizing radiation
![]() The effect of salt
The effect of salt
![]() The effect of temperature
The effect of temperature
![]() The control of activity in foodstuffs
The control of activity in foodstuffs
![]() Colligative properties of water
Colligative properties of water
![]() Overview of colligative properties
Overview of colligative properties
![]() Vapor pressure lowering: CaCl2
Vapor pressure lowering: CaCl2
![]() Freezing point depression: examples: Glucose, Urea, Ethanol, NaCl, CaCl2
Freezing point depression: examples: Glucose, Urea, Ethanol, NaCl, CaCl2
![]() Boiling point elevation: Glucose
Boiling point elevation: Glucose
![]() Osmotic pressure
Osmotic pressure
![]() Osmotic pressure
Osmotic pressure
![]() Osmotic flux
Osmotic flux
![]() Relation of osmotic pressure to vapor pressure lowering
Relation of osmotic pressure to vapor pressure lowering
![]() Osmotic pressure of polymers
Osmotic pressure of polymers
![]() Osmotic potential
Osmotic potential
![]() Solar powered water harvesting
Solar powered water harvesting
![]() Treatment of contaminated water
Treatment of contaminated water
![]() Self-generation of osmotic pressure at interfaces
Self-generation of osmotic pressure at interfaces
![]() Osmotic pressure of particles and membranes
Osmotic pressure of particles and membranes
![]() Exclusion zone (EZ) water
Exclusion zone (EZ) water
![]() Proposal for the generation of osmotic pressure at aqueous interfaces
Proposal for the generation of osmotic pressure at aqueous interfaces
![]() Similarities of EZ-water to osmotically-generated surface water
Similarities of EZ-water to osmotically-generated surface water
![]() Osmotic pressure stabilizes nanobubbles
Osmotic pressure stabilizes nanobubbles
![]() Introduction to aqueous biphasic systems
Introduction to aqueous biphasic systems
![]() Polyoxomolybdate systems
Polyoxomolybdate systems
![]() {Mo132} nanodrop Jmol animation
{Mo132} nanodrop Jmol animation
![]() nanodrop + fullerene, Jmol animation
nanodrop + fullerene, Jmol animation
![]() {Mo154} nanowheel Jmol animation
{Mo154} nanowheel Jmol animation
![]() {Mo132} nanocapsule and aqueous nanodrop
{Mo132} nanocapsule and aqueous nanodrop
![]() The giant Mo240 hollow opening dodecahedra
The giant Mo240 hollow opening dodecahedra
![]() Molybdenum blue {Mo154} nanowheel
Molybdenum blue {Mo154} nanowheel
![]() How does the {Mo154} nanowheel hydrate?
How does the {Mo154} nanowheel hydrate?
![]() How do the nanowheels form large spherical clusters?
How do the nanowheels form large spherical clusters?
![]() Aqueous
solutions of the fullerenes C60 and C70
Aqueous
solutions of the fullerenes C60 and C70
![]() Water surrounding fullerenes
Water surrounding fullerenes
![]() Fullerenes containing water
Fullerenes containing water
![]() Frequently
asked questions concerning liquid water
Frequently
asked questions concerning liquid water
![]() How
can hot water freeze quicker than cold water?
How
can hot water freeze quicker than cold water?
![]() Can
increasing pressure prevent water from freezing?
Can
increasing pressure prevent water from freezing?
![]() Why does salt lower the freezing point of water?
Why does salt lower the freezing point of water?
![]() Does
magnetic descaling of water work?
Does
magnetic descaling of water work?
![]() How
can a liquid have a structure?
How
can a liquid have a structure?
![]() Does
the radial distribution peak at about 3.7
Å exist?
Does
the radial distribution peak at about 3.7
Å exist?
![]() Is
there fine structure in the radial distribution function?
Is
there fine structure in the radial distribution function?
![]() Do
interstitial water molecules exist?
Do
interstitial water molecules exist?
![]() Icosahedral clustering and the two-state
mixture model?
Icosahedral clustering and the two-state
mixture model?
![]() Water-related
material
Water-related
material
![]() Published evidence for and against homeopathy
Published evidence for and against homeopathy
![]() Homeopathic solutions
Homeopathic solutions
![]() Does homeopathy work?
Does homeopathy work?
![]() The placebo effect
The placebo effect
![]() Is water special?
Is water special?
![]() Does the glassware matter?
Does the glassware matter?
![]() Is gas important?
Is gas important?
![]() Does dilution happen as predicted?
Does dilution happen as predicted?
![]() Solutions are more complex than expected
Solutions are more complex than expected
![]() Peroxide and radical production in water
Peroxide and radical production in water
![]() Possible scenarios for the memory effect in homeopathic solutions
Possible scenarios for the memory effect in homeopathic solutions
![]() Polywater, EZ-water and other Waters
Polywater, EZ-water and other Waters
![]() Strange Waters
Strange Waters
![]() IE
IE
![]() HHO
HHO
![]() Neowater
Neowater
![]() Clustered and 'declustered' water
Clustered and 'declustered' water
![]() Fullerane Jmol animation
Fullerane Jmol animation
![]() Novel
fulleranes and carbons
Novel
fulleranes and carbons
![]() Novel fulleranes
Novel fulleranes
![]() C18 and other carbons
C18 and other carbons
![]() Platonic solids Java animation
Platonic solids Java animation
![]() Platonic
solids, water, and the golden ratio
Platonic
solids, water, and the golden ratio
![]() Contributed
papers
Contributed
papers
![]() J.
G. Watterson, Enzyme function: random events or coherent
J.
G. Watterson, Enzyme function: random events or coherent
![]() Book reviews
Book reviews
![]() Handbook of refractive index and dispersion of water for scientists and engineers
Handbook of refractive index and dispersion of water for scientists and engineers
![]() Aqueous systems at elevated temperatures and pressures
Aqueous systems at elevated temperatures and pressures
![]() References 1 - 100 (currently 5392 including multiple references in entries; + ~400 external website references)
References 1 - 100 (currently 5392 including multiple references in entries; + ~400 external website references)
![]() Visitor's
Book, recent postings
Visitor's
Book, recent postings
![]() Visitor's
Book archive, 2000-2003
Visitor's
Book archive, 2000-2003
![]() Visitor's
Book archive, 2004-2006
Visitor's
Book archive, 2004-2006
Home | Site Index | LSBU | Top
This page was established in 2006 and last updated by Martin Chaplin on 12 February, 2023