
Ice IV substructure
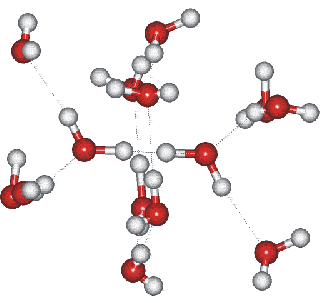
Ice-four (ice IV) may be formed occasionally by the heating high-density amorphous ice at a slow (0.4 K ˣ min−1) rate from 145 K and at a constant pressure of 0.81 GPa [386] (a faster rate, for example, 15 K ˣ min−1, preferably produces ice-twelve). Ice-four is metastable within the ice-three, ice-five, and ice-six phase space (see Phase Diagram). It forms a rhombohedral crystal (Space group, 167; Laue class symmetry -3m1) with cell dimensions 7.60 Å (a, b, c; 70.1°, 70.1°, 70.1°, 16 molecules) [387]. All water molecules are hydrogen-bonded to four others in the crystal,, two as donors and two as acceptors. The structure is formed from a single catenated interpenetrating network of both puckered and flattish hexamers (these allow the penetration) consisting of more strongly hydrogen bound water. a
Ice IV crystal structure
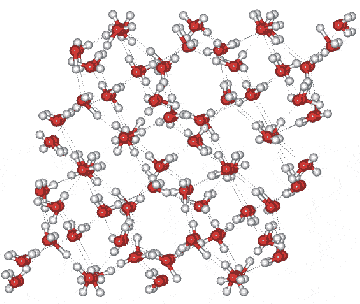
The penetrating hydrogen bond is longer (2.921 Å), but these water molecules also possess three shorter hydrogen bonds (2.783 Å). The hydrogen bonds forming the flattish rings are also somewhat extended (2.876 Å). The networks are not entirely independent as three-quarters of the water molecules have one weaker hydrogen bond to the other network (2.806 Å, 143°); the fourth type of hydrogen bond present. Hence, molecules fall into two unequal classes experiencing different molecular environments. Note that in this structural diagram the hydrogen-bonding is ordered whereas in reality, it is random [460] (obeying the 'ice rules': two hydrogen atoms near each oxygen, one hydrogen atom on each O····O bond). This disorder gives rise to a zero-point entropy close to 3.396 J mol−1 K−1 [2153]. Ice-four is one of four disordered ice crystals (with cubic ice, ice-sixteen, and ice-seventeen) where the ordered form is difficult to produce [4431] and has not yet been definitedly found. The near-infrared spectrum of ice-four has been compared with that of hexagonal ice [4189].
There is a reported triple point between the metastable phases of Ice XII and Ice IV and liquid water at -6 °C and ≈ 500-600 MPa [1300], but this needs confirmation.
Interestingly, ammonium fluoride (NH4+F−), which has a similar hydrogen-bonded hexagonal crystal to hexagonal ice, converts, upon compression at 77 K, to a high-pressure phase isostructural with ice-four. Under the same circumstances, hexagonal ice converts to high-density amorphous ice (HDA) rather than ice-four. This indicates that HDA may be a ‘derailed’ state along the hexagonal ice to ice-four pathway [2861].
Interactive Jmol structures are given.
a The hydrogen-bonded structure of ice-four has been questioned [403], but recent Raman spectra seem to support the structure presented here [460]. It may be that it is more stable as the deuterated ice. [Back]
Home | Site Index | Phase Diagram | Ices, introduction | Ice-Ih | Ice-Ic | Ice-Isd | II | III | V | VI | VII | VIII | IX | X | XI | XII | XIII | XIV | XV | XVI| XVII | XVIII | Amorphous ice | LSBU | Top
This page was established in 2002 and last updated by Martin Chaplin on 20 February, 2022