
Ice-two (ice II) may be synthesized from hexagonal ice at 198 K and 300 MPa or by decompressing ice-five (ice V) at 238 K but is not easily formed by cooling ice-three (ice III) (see Phase Diagram). It may form a significant proportion of icy moons such as Jupiter's Gannymede [839]. Ice II has an ordered arrangement of its hydrogen bonds. Contrary to other ordered ices, ice II does not have a disordered form. On warming towards its melting point, it converts to ices Ih, III, V, or VI. Surprisingly, ice II disappears from water’s phase diagram following the addition of small amounts of ammonium fluoride (for example, 0.5 mol% NH4+ F−) [3260], as a hydrogen-disordering agent.
Ice II unit cell
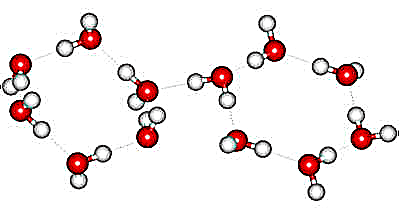
Its unit cell, which forms rhombohedral
crystals (Space group ![]() ), is shown on the right. In the crystal, all water molecules are hydrogen-bonded to four others,
two as donor and two as acceptor, Ice-two may exist
meta-stably below ≈ 100 K between ambient pressure and
≈ 5 GPa. At ambient pressure, it irreversibly transforms
into ice Ic above 160 K. As the H-O-H angle does not vary much
from the isolated molecule, the hydrogen bonds
are not straight (although shown so in the figures). Unexpectedly and due to zero-point energy differences, while the ice II to ice Ic transition is endothermic for H2O (171 K, +40 J ˣ mol−1), it is exothermic for D2O (180 K, −140 J ˣ mol−1) [4132].
), is shown on the right. In the crystal, all water molecules are hydrogen-bonded to four others,
two as donor and two as acceptor, Ice-two may exist
meta-stably below ≈ 100 K between ambient pressure and
≈ 5 GPa. At ambient pressure, it irreversibly transforms
into ice Ic above 160 K. As the H-O-H angle does not vary much
from the isolated molecule, the hydrogen bonds
are not straight (although shown so in the figures). Unexpectedly and due to zero-point energy differences, while the ice II to ice Ic transition is endothermic for H2O (171 K, +40 J ˣ mol−1), it is exothermic for D2O (180 K, −140 J ˣ mol−1) [4132].
Half the open hexagonal channels of ice Ih have collapsed in ice II. The relationship of the ice II structure to ice Ih can be visualized by detaching the columns of hexameric ice Ih rings, moving them relatively up or down at right angles to their plane, rotating them about 30° around this axis and re-linking the hydrogen bonds in a more compact way to give a density of 1.16 g cm−3. The hydrogen bonding is ordered and fixed in ice-two, as can be seen in the linkages around the hexamers. There is no corresponding disordered phase, in contrast to the other ordered ices VIII, IX, XI, and XV. Putative disordered forms of ice II have been modeled and examined but found to be less stable [3463], due to higher Gibbs free energy and zero-point energy. However, the possibility remains of partial hydrogen-bond-disorder in real ice II. The lack of a disordered phase has been correlated with the high energy difference between the most and the second most stable ice configurations [1655]. Some of the ice-two's hydrogen bonds are bent and, consequentially, much weaker than the hydrogen bonds in hexagonal ice.
Ice-two crystal, view down the hexagonal c axis
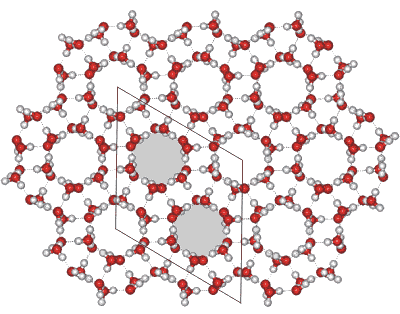
See left for the view down the hexagonal c axis.
The rhombohedral crystal has unit cell dimensions 7.78 Å (a, b, c; 113.1°, 113.1°, 113.1°, 12 mols) [384], each containing 12 water molecules. The unit cell consists of two hydrogen-bonded hexamers, one chair-form (above left) and one almost flat (above right). Two molecules (towards the center bottom in the above diagram) are shown closely approaching (3.23 Å), but they are not hydrogen-bonded. It may be easier to visualize the crystal as hexagonal, each unit cell comprising 36 water molecules (as shown opposite, with dimensions a, b, c; 12.935 Å, 12.935 Å, 6.233 Å; 225 K. 0.25 GPa, [839]). The hexagonal channels (as indicated gray above) form columns of alternating puckered and flat rings going away from the viewer and remain from the conversion from hexagonal ice with the conservation of their hydrogen bonds (3/4 of the total).
Ice-two crystal
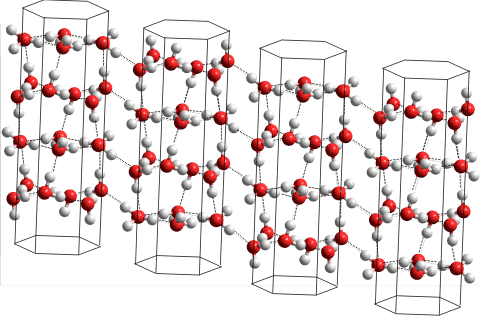
A further 1/3 of the bonds between these channels (1/12 of the total number) are also conserved. The formation of the newly rearranged remaining 1/6 hydrogen bonds found between these channels causes the proton order in ice-two [2479].
The figure opposite shows the view almost perpendicular to the c axis, showing the hydrogen bonding between these hexagonal channels. Single crystals of hexagonal ice may transform into single crystals of ice-two or into crystalline twins of ice two with c axes rotated by 180° to each other [2479]. Up to 7/12 of all hydrogen bonds are retained on the irreversible cooperative ice-two → cubic ice transition [2479].
The experimental 2D IR spectra of D2O isotopically pure ice Ih, ice II, ice V, and ice XIII have been compared [3121]. The near-infrared spectrum of ice-two has been compared with that of hexagonal ice [4189].
Ice-two cannot incorporate NH4F in contrast to hexagonal ice, ice III and other ices [3260]. Ice-two has triple points with hexagonal ice and ice-three (-34.7 °C, 212.9 MPa), ice-three, and ice-five (-24.3 °C, 344.3 MPa) and ice-five, ice-eleven, and ice Ih (-199.8 °C, 70 MPa), and ice-six (estimated at -55 °C, 620 MPa). The relative permittivity (dielectric constant) of ice-two is about 3.7 [94]. The SeaFreeze package allows the computation of the thermodynamic (phase boundaries, density, entropy, specific heat, isothermal bulk modulus, adiabatic bulk modulus, thermal expansivity, chemical potentials) and elastic properties of ice II over the 0-2300 MPa and 220-500K range [3832].
Interactive Jmol structures are given.
Home | Site Index | Phase Diagram | Ices, introduction | Ice-Ih | Ice-Ic | Ice-Isd | III | IV | V | VI | VII | VIII | IX | X | XI | XII | XIII | XIV | XV | XVI| XVII | XVIII | Amorphous ice | LSBU | Top
This page was established in 2001 and last updated by Martin Chaplin on 12 September, 2021