
Ice-seven (ice VII) [1226] forms from liquid water above 3 GPa by lowering its temperature to ambient temperatures (see Phase Diagram). Also, water can freeze to ice VII on sub-microsecond timescales under dynamic compression [3280]. Ice-seven has been found in inclusions in diamonds [3251], in the Earth's mantle, and the solar system planets and icy moons. Using laser-induced shock waves (≈ 5 GPa), liquid water transforms almost immediately (≈ 10 ns) to ice VII [2976]. It may be considered the most stable phase as it exists over a wide temperature and pressure range. It is a plausible constituent of giant planets and icy moons. It can be obtained at low temperature and ambient pressure by decompressing (D2O) ice-six below 95 K and is metastable over a wide pressure range, transforming into LDA above 120 K [948]. It can also be formed using picosecond laser pulses (causing high pressure, ≈ 10 GPa, shock waves) at 532 nm and 1064 nm wavelengths [2688]. The bulk modulus and equation of state of ice VII have been described [3300].
Ice VII substructure
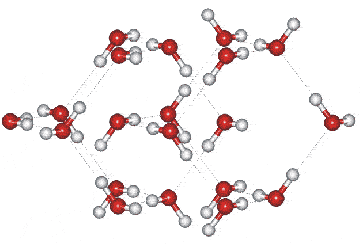
The Ice VII unit cell, which forms a cubic crystal
(![]() , 224; Laue class symmetry m-3m) consists of two interpenetrating cubic
ice lattices with hydrogen bonds passing through
the center of the water hexamers and no connecting hydrogen bonds
between lattices. It has a density of about 1.65 g cm−3 (at 2.5 GPa and 25 °C [8]),
which is less than twice the cubic ice density as the
intra-network O····O distances
are 8% longer (at 0.1 MPa) to allow for the interpenetration.
The cubic crystal (shown on the right) has cell dimensions
3.3501 Å (a, b, c, 90º, 90º, 90º;
D2O, at 2.6 GPa and 22 °C [361])
and contains two water molecules.
, 224; Laue class symmetry m-3m) consists of two interpenetrating cubic
ice lattices with hydrogen bonds passing through
the center of the water hexamers and no connecting hydrogen bonds
between lattices. It has a density of about 1.65 g cm−3 (at 2.5 GPa and 25 °C [8]),
which is less than twice the cubic ice density as the
intra-network O····O distances
are 8% longer (at 0.1 MPa) to allow for the interpenetration.
The cubic crystal (shown on the right) has cell dimensions
3.3501 Å (a, b, c, 90º, 90º, 90º;
D2O, at 2.6 GPa and 22 °C [361])
and contains two water molecules.
All molecules experience identical molecular environments. The hydrogen-bonding is disordered and continually changing as in hexagonal ice but ice-seven undergoes a proton disorder-order transition to ice-eight at about 5 °C; ice-seven and ice-eight having identical structures apart from the proton ordering. Ice-seven is metastable indefinitely at 77 K.
Pressure volume data for ice VII, from [1943]
![Pressure volume data for ice VII at room temperature, from [1943] Pressure volume data for ice VII at room temperature, from [1943]](images/ice_VII_pressure.gif)
If the pressure is increased above about 14 GPa there are changes in the proton ordering [1428]. This is shown in the phase diagram and gives rise to a loss of the cubic symmetry [2126]. This has been thought due to some dissociation with some hydrogen ions occupying the octahedral cavities, eventually rising at greater than 30 GPa to plateau at one interstitial occupancy to every two water molecules [2027a]; a this is equivalent to two of each six positions (as shown below right) being occupied. These interstitial protons cannot form normal hydrogen bonds with the H2O oxygen atoms. Molecular dynamics and modeled X-ray Raman spectroscopy (XRS) spectra did not show such extensive interstitial occupancy [2376], so the exact structure remains somewhat unresolved. Dissociation is responsible for the peak in the electrical conductivity at about 10 GPa during compression of D2O from 5.5 GPa to 17 GPa between 400 K and 425 K and due to the increase in H3O+ and OH− charge carriers [2244]. Neutron diffraction data to 15 GPa has shown that the rate of hydrogen ordering at the ice VII-VIII transition decreases strongly with pressure to reach timescales of minutes at 10 GPa, but the ordering process becomes more rapid again upon further compression. This has been explained by a slowing down of the rotational dynamics of water molecules with a simultaneous increase of translational motion of hydrogen under pressure, and is likely to be the origin of various hitherto unexplained anomalies of ice VII in the 10-15 GPa range reported by Raman spectroscopy, X-ray diffraction, and proton conductivity [3909]. At higher pressures, ice-seven undergo a continuous transition into cubic ice-ten (ice X) where most hydrogen atoms are situated midway between the oxygen atoms. Neutron-diffraction of deuterated ice VII up to over 60 GPa shows a clear elongation of the O-D covalent bond, increasing with pressure [4226]. The size of the ice crystal (two molar volumes per unit cell) continuously reduces over this large change in pressure with no obvious volume steps at the expected ice transitions [1943].
Partial structure of ice VII showing interstitial sites
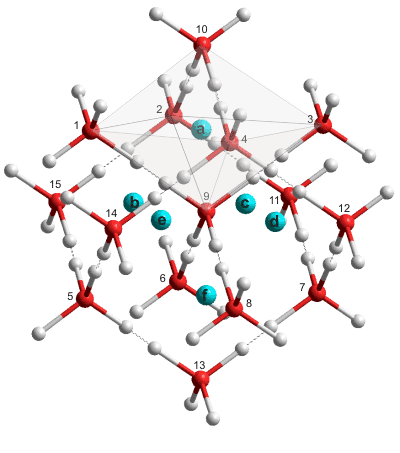
Shown opposite is a cartoon of ice-seven showing the octahedral interstitial cavities (shown as blue spheres a-f). The water molecules are shown with the positions of their four possible hydrogen atoms, although only two, or less, would be occupied obeying the 'ice rules'. Any interstitial hydrogen atoms (shown here labeled a-f) sit in the six octahedral cavities and are centered on the body-centered cubic square faces. As examples, an octahedral cavity (a, shown gray) is made from the water molecules 1, 2, 3, 4, 9, and 10 and sits in the square face made from the water molecules 1, 2, 3 and 4 of the body-centered cube centered on water molecule 9. Also, cavity (e) is formed from the water molecules 1, 4, 5, 8, 9, and 14 and in the square face made from the water molecules 1, 4, 5, and 8 of the body-centered cube, and cavity (f) is formed from the water molecules 5, 6, 7, 8, 9, and 13 and in the square face made from the water molecules 5, 6, 7, and 8 of the body-centered cube.
In contrast to ice Ih, high-density ices may incorporate some solutes into their crystal structure [3582 ]. Ice-seven may incorporate up to ≈ 7.5 wt % NaCl into the octahedral cavities (face-centered positions of its body-centered cubic structure, described above right) at high pressure (for example, 4-21 GPa) [955]. Counter-intuitively, this incorporation causes a reduction in the unit cell dimensions and a greater than expected increase in density [955]. The inclusion of salt also raises the pressure required for conversion to ice X [2373].
Ice-seven has known triple points with ice-six and ice-eight (5 °C, 2.1 GPa), ice-eight, and ice-ten (100 K, 62 GPa) and liquid water and ice-six (355 K, 2.216 GPa). Ice-seven pressure-volume data has been described [2337]. Interestingly, at high pressures (≈ 2.3 GPa), liquid water can be made to freeze at over 100 °C (to give the denser ice-seven). The relative permittivity (dielectric constant) of ice-seven is about 150. Its dielectric responses, under a pressure of 10 GPa and at a temperature of 410 K, to applied oscillating electric fields of various frequencies have been investigated, using non-equilibrium ab initio molecular dynamics. [4371]. High static electric fields have a greater effect and may lead to the electro-freezing of ice-seven, even causing it to transition to the superionic phase by promoting the O-H dissociation [4372].
Although there is no experimental evidence, molecular dynamics studies predict the existence of plastic water ices at the interface between ice VII and liquid water. In these theoretical plastic phases, the H2O molecules freely rotate around fixed lattice sites [3509].
Interactive Jmol structures are given.
a This explanation is disputed after re-examination using X-ray Raman spectroscopy [2027b]. [Back]
Home | Site Index | Phase Diagram | Ices, introduction | Ice-Ih | Ice-Ic | Ice-Isd | II | III | IV | V | VI | VIII | IX | X | XI | XII | XIII | XIV | XV | XVI| XVII | XVIII | Amorphous ice | LSBU | Top
This page was established in 2002 and last updated by Martin Chaplin on 14 November, 2021