
Ice-ten cubic structure consisting of 8 unit cells of two water molecules
plus extra atoms found within the basic cubic structure
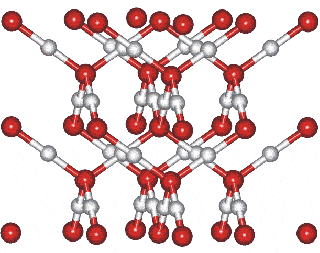
Ice-ten lattice
The ice VII - ice X phase line has been described by an equation of state covering pressures up to 450 GPa [3860] but varies between studies [4223]. The melting curve for ice-ten has been proposed at high temperatures (1000-2400 K) [612a]. As ice X is approached with increasing pressure from very hot liquid water and the O-H energy minima draw near each other, the protons rapidly swap positions giving many short-lived OH− and H3O+ ions [612b]. In ice X, however, protons are less mobile than in ice VII as there is only one energy minimum for each O-H-O bond. The bulk modulus of ice X has been established as about 600 GPa at a pressure of 120 GPa [4225].
Ice-ten looks identical as seen from the x-, y- or z-direction. Using a first-principles DFT code, ideal crystals of ice X have been shown to have a Raman peak at 998 cm−1 and two infrared absorption peaks at 450 cm−1 and 1507 cm−1 [3481].
Ice-ten has triple points with ice-seven and ice-eight (100 K, 62 GPa) and ice-seven and water (≈ 1000 K, ≈ 47 GPa) [612b].
It has been theoretically determined that doping ice-ten with NH2 gives rise to a superconductor with a critical temperature of about 60 K at 150 GPa [2990].
Interactive Jmol structures are given.
Home | Site Index | Phase Diagram | Ices, introduction | Ice-Ih | Ice-Ic | Ice-Isd | II | III | IV | V | VI | VII | VIII | IX | XI | XII | XIII | XIV | XV | XVI | XVII | XVIII | Amorphous ice | LSBU | Top
This page was established in 2003 and last updated by Martin Chaplin on 4 October, 2021