
Methane clathrate hydrate I
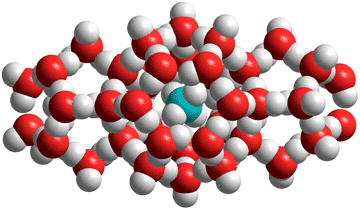
Clathrate hydrates are solid cages of water containing small nonpolar guest molecules like carbon dioxide and methane.
![]() CS-I clathrate hydrate
CS-I clathrate hydrate
![]() CS-II clathrate hydrate
CS-II clathrate hydrate
![]() HS-III clathrate hydrate
HS-III clathrate hydrate
![]() Other structures
Other structures
![]() The Gibbs phase rule
The Gibbs phase rule
"The solution of oxymuriatic gas (chlorine) in water freezes more readily than pure water"
Sir Humphrey Davy 1811; the first reference to a clathrate a
The nucleation and crystal growth of the gas hydrates have been described [3581], with insights into their thermodynamics provided [3581]. Clathrate hydrate crystalline ices b form from water and non-stoichiometric amounts of small nonpolar molecules (hence usually gaseous) under moderate pressure (typically of a few MPa) and at cold temperatures (typically close to 0 °C, but increased pressure raises the melting point). They may be treated as solid solutions of volatile solutes and generally involve higher solubilities in the solid-state relative to the liquid state. The water molecules are linked through hydrogen-bonding and form complex lattice-like structures with substantial cavities. All clathrate hydrates contain convex cavities as the concave puckered structures undergo rapid rearrangement. These rearangements are the reason why no crystalline aqueous clathrate structures contain water molecules in their cavities.
Clathrates are notable due to their hazardous formation in natural gas pipeline blockages, their occasional hazardous release of large volumes of gas from underwater natural reservoirs, their potential for the production of natural gas from deep-sea sources, and their potential in the removal of carbon dioxide (the global-warming gas) from the atmosphere. Some physical properties of the common gas hydrates have been reviewed [3310]. All clathrate hydrates have quadruple points c where the vapor, hydrate, ice, and aqueous solution are in equilibrium. For argon, this is at 272.2K and 8.267 MPa [3427].
Clathrate hydrates are in equilibrium with their free guest species with their occupancy dependant on the temperature and pressure. Clathrate hydrates are unstable, at positive pressure [520], in the absence of the solute. Every water molecule forms a vertex of four cages, which may or may not, contain a small guest molecule. Their structures require a minimum amount of these small molecules to fit into and stabilize the cavities (usually one or none in each cavity) without forming any covalent or hydrogen bonds to the water molecules. The cavities obey Euler's convex polyhedra theorem:
Faces - Edges+ Vertices = 2
There are van der Waals interactions between the guest molecules and the surrounding cages. However, the critical factor in the cages' stabilities is that the guest molecules prevent their collapse. Without these interstitial molecules, the clathrate cavities (shown below) would collapse at positive pressures, and they then dissipate, if surprisingly slowly, after the clathrate ice melts [897]. During formation and dissociation, the solid clathrates interact significantly with the structure of the neighboring aqueous solution [904]. Gas hydrates may slowly dissociate below their melting point with the release of gas and surface ice [2690]. The hydrogen-bondings in the clathrate ices are oriented in an imperfectly disorderly manner obeying the 'ice rules' [1990]. The relationships between the molecular properties of various hydrate-forming gas species and the thermodynamic stability of the associated clathrate hydrate systems have been investigated [2823].
CS-I and CS-II are the most stable structures, and no other hydrate structure with a single guest component has been found under ambient conditions (except for bromine clathrate) [1732]. The lattice constants and expansivities of gas hydrates CS-I and CS-II have been described from 10 K up to the stability limit [3313]. CS-I has 46 H2O per unit cell in three non-equivalent sites with 6, 16 and 24 H2O, average O····O distance of 2.793 Å and average departure of the O··O··O angle from tetrahedral of 3.7°. CS-II has 136 H2O per unit cell in three non-equivalent sites with 8, 32 and 96 H2O, average O····O distance of 2.790 Å and average departure of the O··O··O angle from tetrahedral of 3.0°. The structure and thermodynamics of empty clathrate hydrates below the freezing point of water have been described [4291.
It has been proposed that the HS-IQ clathrate (with a maximum 0.175 guest/H2O) structure may be an intermediate in the gas pressure transition of CS-I (maximum 0.174 guests/H2O) to CS-II (with a maximum 0.176 guest/H2O) [1870].
Methane clathrate initiation
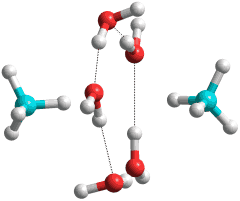
He-clathrates can be formed by filling empty clathrate (and some ice) structures [3963], including helium entering into ice Ih [3320]. Other clathrate hydrates are initiated [3309] by van der Waals linked molecules (guests) situated next to cyclic hydrogen-bonded water rings (host) at the gas-liquid boundary. For example, methane clathrates start with a water pentamer combined with one or two water molecules (see right [3301]). Investigation of the formation of methane clathrate has shown that their growth starts with one pentagonal ring of water molecules with one methane molecule for the small cages and one hexagonal ring of water molecules with one methane molecule for the large cages [2813]. Using surface-specific sum-frequency vibrational spectroscopy, the nucleation and disintegration of methane clathrate embryos at the methane-gas-water interface has been studied [3803]. The interfacial methane undergoes three steps under high pressure and at different temperatures; (a) dissolution of the methane molecules at the water interface, (b) formation of the caged methane–water complexes, and (c) the appearance of microscopic methane clathrate crystals that act as nuclei for clathrate growth. Throughout, the bulk water structure remains unchanged. Ternary water-ring clusters with three inter-connected five-, six-, and seven-membered rings have been identified as the fundamental structures that characterize the nucleation pathway. Clusters of these dehydrate (shed the excess water molecules) to form a critical clathrate nucleus of about 13 methane molecules with about 100 water molecules [4087].
Methane prefers to stay at the 512 cavities in comparison to the 51262 cavities by 5.4 kJ ˣ mol−1 and stabilizes the 512 cavities by 7.2 kJ ˣ mol−1 [3116]. There is espeedy methane diffusion at the interface of clathrate structures I and II at 0.8 GPa. d This may be due to the formation of micro-/nano-bubbles at the interface between the two snetworks [3312]. At 0.8 GPa, CS-II is not a stable phase of methane clathrate hydrate but a persistent metastable state that forms dependent on the preparative methodology.
Type (in order of increasing energy of empty network) |
Lattice |
Space group |
Unit cell |
Unit cell formula a |
H2O/guest, min |
|---|---|---|---|---|---|
| Clathrate II, CS-II | Face-centered cubic | Fd3m |
a=1.731 nm | (S)16(L+)8·136H2O |
136/24 = 5.67 |
| Clathrate I, CS-I | Cubic | Pm3n |
a=1.203 nm | (S)2·(L)6·46H2O |
46/8 = 5.75 |
Clathrate TS-I, [1734] Clathrate III |
Tetragonal | P42/mnm |
a=2.318 nm c=1.215 nm |
(L)12-16
·(L+)4 |
172/20 = 8.6 |
| Clathrate HS-IQ [1869] | Hexagonal | P6/mmm |
a=1.1987 nm c=1.1509 nm |
(S)3·(L)2(L+)2·40H2O |
40/7 = 5.71 |
| Clathrate H, HS- III | Hexagonal | P6/mmm |
a=1.23 nm c=1.02 nm |
(S)5(L++)·34H2O |
34/6 = 5.67 |
| Clathrate S- III [2507] | Cubic | a=1.3431 nm |
(L++)2·48H2O |
48/2 = 24 |
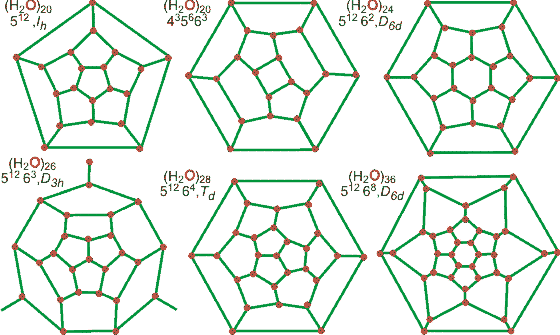
512 |
51262 |
51263 |
51264 |
51268 |
435663 |
|
|---|---|---|---|---|---|---|
H2O |
20 |
24 |
26 |
28 |
36 |
20 |
Mean cavity radius, Å |
3.95 |
4.33 |
4.53 |
4.73 |
5.71 |
4.06 |
Major axis, Å, to atoms |
4.84 |
6.12 |
- |
6.29 |
8.44 |
4.96 |
Minor axis, Å to atoms |
4.84 |
5.07 |
- |
6.29 |
6.80 |
4.26 |
free volume, Å3 |
51 |
77 |
98 |
120 |
213 |
44 |
Faces |
12 |
14 |
15 |
16 |
20 |
12 |
Edges |
30 |
36 |
39 |
42 |
54 |
30 |
Vertices |
20 |
24 |
26 |
28 |
36 |
20 |
H2O per cavity |
5 |
6 |
6.5 |
7 |
9 |
5 |
| CS-I, /unit cell | 2 |
6 |
- |
- |
- |
- |
| CS-II, /unit cell | 16 |
- |
- |
8 |
- |
- |
| HS-III, /unit cell | 3 |
- |
- |
- |
1 |
2 |
| TS-I, /unit cell | 10 |
16 |
4 |
- |
- |
- |
| HS-IQ, /unit cell | 3 |
2 |
2 |
- |
- |
- |
| Guest molecules, for example; approximate radius, Å |
Ar, H2, O2, N2, CO2, CH4, Ne, Cs+, H2S |
CO2, C2H6, Xe, (H2)2, He4, H2S |
Br2 |
C3H8, (H2)4,(CH3)3CH, (H2S)1-2 |
(CH3)3CC2H5, Xe, (H2S)1-4, adamantane |
CH4, H2S |
1.8-2.2 |
1.8-2.7 |
≈ 2.4 |
2.8-3.1 |
3.5-4.3 |
1.8 |
Cage structures
a Not all cavities would normally be filled; S = small guest; L = large guest; L+ = larger guest; L++ = largest guest
The 512 cavity (H2O)20, forming a spheroidal pentagonal dodecahedron of radius 3.86 Å, is present in all known clathrate hydrate structures. Slightly less stable, by 3.8 kJ ˣ mol−1, is the 435663 cavity (H2O)20, where the average cavity radius is marginally larger (computed 3.91 Å, experimental 4.06 Å). The oblate ellipsoidal tetrakaidecahedron 51262 cavities (H2O)20 is the second most common unit with short 4.03 Å and long 4.48 Å radii, able to host larger molecules. The larger spheroidal 51264 hexakaidecahedron (H2O)28 has an average radius (4.62 Å) close to the mean cavity radius (4.73 Å) found in the sII clathrate structure [4021].
Connectivity maps (Schlegel diagrams) for the clathrate cages
Some clathrate hydrates can form, at atmospheric pressure, at the interface between a liquid of suitable guest molecules and water (for example, CH3CCl2F in clathrate CS-II hydrate [408]). At low pressures (e.g., atmospheric), most clathrate hydrates decompose to release the guest molecules, except at low temperatures (for example, < 270 K), where they may remain in a metastable state, for several hours. At very high pressures, clathrate hydrates show complex phase behavior, often giving filled hexagonal ice [1144 ] with the smaller guest molecules/atoms, then at even higher pressures, they break down to give high-density ice and a solid phase formed by the guest molecules (for example, see [898]). Gas hydrates have been reviewed [395]. Water itself cannot be contained within the cavities of solid clathrates [1114].
The water oxygen–halogen atom distances in Cl2 clathrate hydrates and Br2 clathrate hydrates are shorter than the sum of the van der Waals radii, showing new non-covalent interactions between the dihalogen guests and the water molecules of the clathrate hydrate frameworks [3153].
The relative content of the cavities can be determined by techniques such as Raman spectroscopy or NMR [3188], as the different cavities present differing environments. [Back to Top ![]() ]
]
Clathrate-I crystal structure
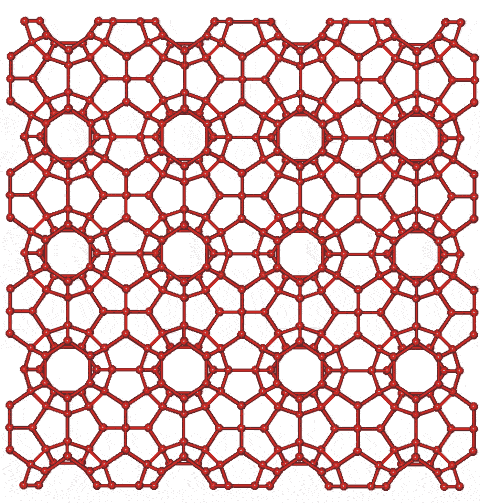
For interactive Figures, see Jmol
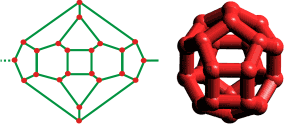
Shown opposite is the cubic clathrate CS-I network formed by small nonpolar (gaseous) molecules, such as CH4 and CO2, in aqueous solution (for example, (CO2)8-y·46H2O) under pressure and at low but not necessarily (normally) freezing temperatures (only the oxygen atoms of water are shown). The included molecules randomly occupy many of the cavities, dependent on their size. Linear tetrakaidecahedral (51262) cavities form three orthogonal axes holding a dodecahedral cavity wherever they cross (ratio 6:2 respectively per unit cell); each dodecahedral cavity is sitting (in a body-centered cubic arrangement) within a cube formed by six tetrakaidecahedral (51262) cavities. These (51262) cavities join at their hexagonal faces to create columns, going away from the viewer in the figure.
This clathrate contains small rings of hydrogen-bonded water molecules containing 5, 6, and 10 molecules in the ratio of 24:3:6, respectively.
Acids such as HClO4 also form CS-I clathrates with the anion in the cages and the excess protons causing occasional vacancies in the clathrate structure [2684].
Burning methane clathrate,
from USGS

Methane hydrate resources from USGS
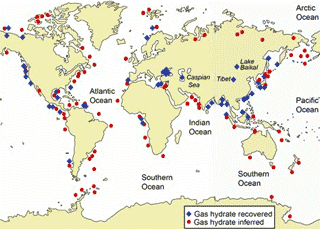
About 6.4 trillion (that is, 6.4 ˣ 1012) metric tons of methane lies at the bottom of the oceans in the form of its clathrate hydrate [899]. More lies in the permafrost. This dissolved carbon is greater than that of all conventional fossil fuels on Earth. Methane is mostly of microbial origin forming in sediments at the edges of continental shelves. They form when enough methane is present to fill ≈ 90% of the cages. Each kilogram of maximal occupied hydrate (only a maximum of about 96% occupancy is found) holds about 190 liters of methane (at atmospheric pressure and ambient temperature). As shown left, this ice will burn and could provide energy equal to over twice the world reserves of natural gas. Exploitation, however, presents many challenges [2557], with China leading the way [2988]. Clathrates are being investigated for efficient and effective natural gas storage and transport [3396, 4463].
Phase diagram for methane hydrate, showing ocean depths
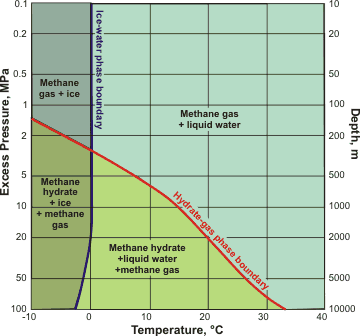
The phase diagram for methane hydrate is shown right [2283] (see also [3587]) but note that the salts present shifts the phase boundary to the left and CO2 shifts it to the right. The main problems facing commercial exploitation are the development of safe and commercially viable technology for extracting methane gas from hydrate deposits. One possible method is to exchange the CH4 with CO2 [3752]. However, if this methane is not successfully mined, global warming may melt this methane hydrate, and vast amounts of methane will be released into the atmosphere. There is an area of the phase diagram above the hydrate-gas-phase boundary shown opposite (i.e., at lower pressure) where the clathrate is metastable but persists for anomalously long periods along with gas and supercooled water. Generally, hexagonal ice is absent, but if eventually present causes dissociation [2814] (the so-called 'self-preservation' effect).
Under higher pressure, CS-I methane hydrate transforms to the CS-II phase at about 120 MPa, and then to the HS-III phase at about 600 MPa before decomposing at pressures above 3 GPa to form solid methane and ice VII [2380] or filled ice structures [3672]. Thus, the CS-II phase is likely the dominant phase within the deep hydrate-bearing sediments underlying continental margins [2380]. Theoretically, above 40 GPa, a new phase is formed (MH-IV) by six-fold water rings (as in hexagonal ice) enclosing the strongly constrained but ordered methane molecules [3850]. Methane hydrate (MH-IV) has been found experimentally at 33.8–57.7 GPa [4211]. It is stable up to at least 134 GPa without decomposition into solid methane and high-pressure ices.
Conversion of CO2 to its clathrate has been investigated as a way of removing the global warming gas CO2 from the atmosphere. It is formed on seabeds and may be created relatively quickly. It is highly unusual in that it decomposes at less-cold temperatures (> 5 °C) and if its temperature is lowered below -150 °C. The stability range is due to the rotational freedom of CO2 above -150 °C with fixed orientation causing clathrate shell distortion below -150 °C [2901].
Tetragonal clathrate TS-I, found in bromine hydrate, has a structure related to CS-I [4091]. Its formation requires the presence of particular guest molecules occupying several cages.
[Back to Top ![]() ]
]
Clathrate-II crystal structure
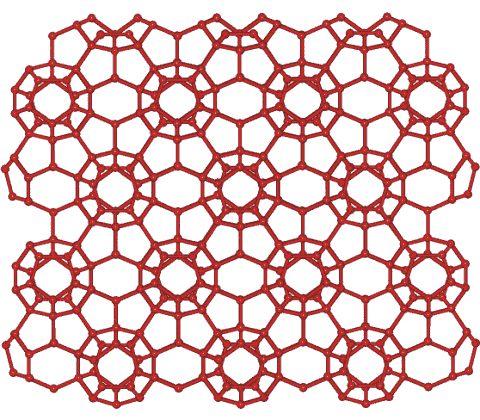
For interactive Figures, see Jmol
Shown opposite is the CS-II hydrate structure (cubic crystals containing sixteen 512 cavities, eight larger 51264 cavities, and 136 H2O molecules per unit cell, and containing larger molecules such as 2-methylpropane in the larger cavities only). The tetrahedral 51264 (H2O)28cavities form an open tetrahedral network, with their centers arranged reminiscent to the cubic ice structure and separated by groups of three 512 cavities. This structure is layered with 512 cavities between layers formed of a mixture of 512 and 51264 cavities. The large proportion of 512 cavities is thought responsible for the similarities in the Raman spectra to gas saturated water [831].
This clathrate contains small rings of hydrogen-bonded water molecules containing 5, 6, and 10 molecules in the ratio of 18:2:12, respectively.
The nucleation and growth of propane clathrate CS-II hydrate have been described, using molecular dynamics simulations, involving the transitory formation of two new (435661 and 425861) cages [3943].
Alkali hydroxide doping can lead to a low-temperature orientational ordering of the hydrogen bonds [2865].
Rather surprisingly, the CS-II clathrate forms with molecular hydrogen (H2), four molecules sitting in the large cages, and one [1257b] or two [1257a] in the small cages, that is a maximum of (2H2)16.(4H2)8.136H2O. [1257a]. This maximum occupancy cannot be reached however, with difficulty forming the doubly-occupied small cages.
Clathrate-II crystals collapse into an (unannealed) high-density amorphous state near 1 GPa on isothermal pressurization below 140 Kn similarly to the collapse of ice Ih to high-density amorphous ice [3732].
[Back to Top ![]() ]
]
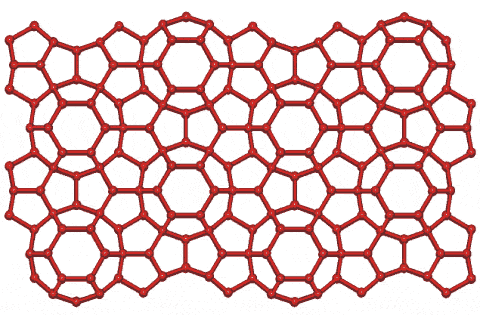
Clathrate-H crystal structure
For interactive Figures, see Jmol
Shown opposite is the HS-III hydrate structure. It has hexagonal crystals containing three 512 (H2O)20 cavities, two small 435663 (H2O)20 cavities, one large 51268 (H2O)36 cavity, and 34 H2O molecules per unit cell,. It contains even larger molecules, such as 2,2-dimethylbutane, in the larger cavities only. These large cavities are stabilized by the presence of smaller molecules in the small cavities. Each 51268 barrel-shaped cavity is surrounded by six 435663 cavities around its central ring of 6 hexagons. These (51268) cavities join at their top and bottom hexagonal faces to form columns, going away from the viewer in the figure. Thus, this structure can be thought of as a layered structure with layers of 512 cavities between layers composed of a mixture of 435663 and 51268 cavities.
This clathrate contains small rings of hydrogen-bonded water molecules containing 4, 5, 6, and 10 molecules in the ratio of 3:30:7:18, respectively.
[Back to Top ![]() ]
]
Connectivity maps for the clathrate cages
of SIII clathrate
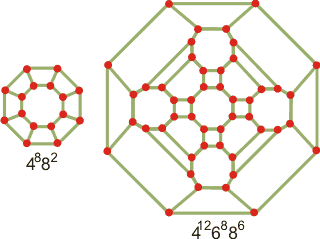
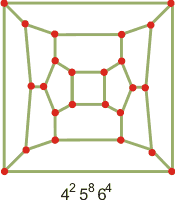
Many other cages and tiled three-dimensional structures are possible [3311] (see in JavaView), and other clathrate structures are being discovered, some related to the Frank-Kasper (FK) structures [1733]. Many other cage structures are allowed in liquid water surrounding small hydrophobic molecules [4373]. They may be complete or incomplete. Thus, there is a tetragonal sT clathrate (space group P42 mnm, made up of only 425864 (H2O)24 cavities with 12 water molecules per unit cell), a tetragonal S-K clathrate (made up of 6 ˣ 512 (H2O)20, 4 ˣ 51262 (H2O)24 and 4 ˣ 51263 (H2O)26 cavities with 80 water molecules per unit cell), and a cubic S-III clathrate (made up of 2 ˣ 4126886 (H2O)48 and 6 ˣ 4882 (H2O)16 cavities with 48 water molecules per unit cell, see also elsewhere) [2507]. Each sT cage can accommodate up to two guest species, resulting in the maximum water: guest ratio of 3:1. The connectivity maps for the clathrate cages in the cubic S-III clathrate are shown left.
Connectivity map for the
clathrate cage of sT clathrate
Some materials can form a range of clathrate hydrates, dependent on their hydration ratio. For example, tetramethylammonium hydroxide (TMA+OH−) forms a range of solid hydrates TMA+OH−.(H2O)n with n = 2 (α and β), 4, 5 (α and β), 7.5 (α and β) and 10 [97b]. The β-pentahydrate has structure 2[4668].10 H2O.2 TMA+OH2, the β-7.5 hydrate 8[51263].4[4258].60 H2O.8 TMA+OH− (the [51263]occupied by the TMA+ with the [4258] unoccupied) and the decahydrate, 4[4151066].4[4356].40 H2O.4 TMA+OH− (the [4151066] occupied by the TMA+ with the [4356] unoccupied); all four-connected throughout, despite their proton deficiencies and with the rare weak hydrogen bond of the type OH−···OH2. The α-7.5 hydrate has the depleted structure 2[4159(5)164(6)1].2[4256(5)263(6)2].2[42(4)155(5)1].30 H2O.4 TMA+OH− with some oxygen atoms being three-connected only (incomplete faces are shown bracketed) [97b].
Connectivity maps for the clathrate cages
of sVI clathrate
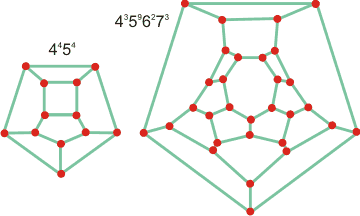
The sVI clathrate of tert-butylamine contains twelve small 4454 cages to every sixteen larger 43596273 (H2O)30 cages (unit cell 156 H20; 16 t-BuNH2), forming a cubic crystalline structure where t-BuNH2 occupies the large cages and the small cages remain vacant. It contains square, pentagonal, hexagonal, and heptagonal ring structures. This clathrate has received much interest due to its ability to absorb up to 7.2% H2 molecules. H2 is found at the shared heptagonal faces of the large (43596273 (H2O)30) cage (withthe t-BuNH2 present) and in cavities formed by the disruption of smaller (4454 (H2O)12) water cages [3302].
The sL clathrate is stable below ≈ -500 MPa with an ultra-low density (0.58 g ˣ cm−3). It contains tiny water cube cavities (46 (H2O)8, 3 ˣ per unit cell), as well as 4668 (H2O)24 (one ˣ per unit cell) and very large cavities 4126886 (H2O)48 (1.296 nm diameter, one ˣ per unit cell) [3316].
Molecular hydrogen (H2) forms several different clathrates as the H2 molecules can fit in most cages with multiple H2 in the larger cages (see above). At high pressures (about 400 MPa, 280 K), a different network structure forms (a filled ice rather than a clathrate) with the composition (H2O)2H2 and consisting of interpenetrating spiral chains of water molecules showing topological similarity to the mineral quartz [2773].
Compression of CO2-clathrate hydrates gives rise to a range of products [3059], including solid H2CO3 and dry ice (solid CO2). A CO2-filled ice is formed from the CS-I hydrate [3059c]. In contrast, CS-II gives a chiral structure [3059b], similar to ice-XVII, which has large open spiral channels containing the freely-moving CO2 guest molecules (1:3.5 CO2: H2O, compared with 1:2 H2: H2O for the pre-ice-XVII structure). It has been suggested that ice XVII may be used as a cheap, useful, and environmentally friendly, microporous material for the storage of CO2. Ice XVII can also be filled with N2 forming an sX clathrate hydrate [3750].
4151062 (H2O)22 cavity
Conversion between clathrate structures may make use of such structures as intermediate, with the 4151062 (H2O)22 cluster (see right) being proposed in the interconversion of CS-I and HS-III clathrates [2384].
[Back to Top ![]() ]
]
a H. Davy, The Bakerian Lecture: On some of the combinations of oxymuriatic gas and oxygene, and on the chemical relations of these principles, to inflammable bodies, Philosophical Transactions of the Royal Society, 101 (1811) 1-35. [Back]
b The 'clathrate' term (clathratus; grated, latticed) was first introduced by H. M. Powell, The structure of molecular compounds. Part VI. Clathrate compounds, Journal of the Chemical Society, (1948) 61-73. [Back]
c The Gibbs phase rule. The Gibbs phase rule states that if a system consists of C components and P phases existing in equilibrium, the number of degrees of freedom (F) is given by F = C − P + 2. Under the singular conditions of temperature and pressure where the aqueous solution, vapor, hexagonal ice, and hydrate are in equilibrium and stably coexist (P = 4), there is a 'quadruple point' (F = 0) for a double component system (C = 2), like the clathrate system. [Back]
d Coincidentally the CS-I – HS-III transition pressure, [3315]. [Back]
Home | Site Index | Water clusters based on clathrate structures | Magic number clathrates | Phase diagram | Ices, introduction | LSBU | Top
This page was established in 2003 and last updated by Martin Chaplin on 20 June, 2022