
The H3O+(H2O)20 cluster
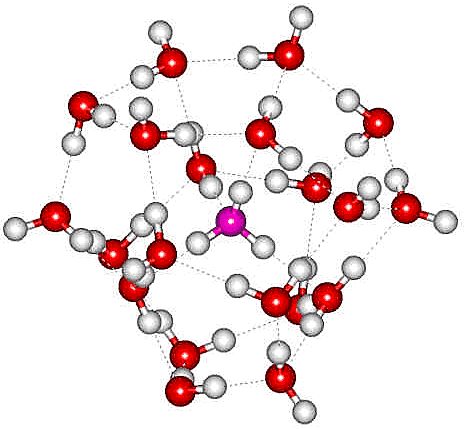
An oxonium ion (H3O+), ammonium ion (NH4+) or alkali metal ion, including Cs+, Rb+, and K+, may sit in the tetrahedral cavity of a collapsed water dodecahedron so forming the magic number cluster [3998]. H3O+(H2O)20 m/z 379 (as opposite with the oxonium oxygen colored magenta, a or NH4+(H2O)20 m/z 378) as found by electrospray mass spectrometry [113] (a similar structuring is found in H2O(H2O)20, the minimal network of surrounding water molecules required to make the central one display the same vibrational features of liquid water [4254]). Infrared studies confirm the dodecahedral clustering by showing that all the dangling OH groups arise from similarly situated water molecules [113c]. It should be noted that the extra proton does not need to be associated with the central water molecule in the above structure. It could hop to any of the other 20 water molecules, and this would be expected in an isolated cluster where the poor hydrogen-bond donation to H3O+ is avoided. Such a surface protonated structure has been confirmed and found to be retained at higher temperatures when two-coordinated water occurs [774]. Such magic number ions are followed by a reduced mass peak at the next H2O addition, an anti-magic number [1918] due to a weak attachment of an extra H2O to the magic number ion. Using a quantum investigation employing semiclassical spectroscopy, the minimum structure responsible for proper solvation of a neutral water molecule (H2O) resulted in a cluster of 21 water molecules as above right but with the central molecule H2O rather than H3O+ [4406].
H3O+(H2O)20 cluster ions have calculated dipole moments of about 10 D (compare H2O dipole 1.86 D) and may contribute to the intense terahertz emission of water vapor under optical excitation [1868]. Anti-magic number clusters, where the cluster is particularly unstable, have been found where the water cluster has one more water molecule than the magic number ion [1995]. This is due to the single extra water molecule being tagged onto the outside of the stable cluster and easily removed from it.
Interactive Jmol structures are given.
This symmetric structure was not as found in an ab initio search [114] but might be expected to be more stable than the one found there as the bond distortion is less and it has one fewer non-hydrogen-bonding hydrogen atom.b The symmetric clathrate structure, containing a single water molecule, is found as the (H2O)21 global minimum energy structure using the TIP5P model [857]. In contrast, the (H2O)20 global minima found using other computational models such as the TIP4P model [115], or using density functional methods [2289], are very different and mainy consist of four-membered rings. Although such low-energy structures (e.g., the structure formed by four fused cubes) are sometimes found as global minima, they have weaker hydrogen bonds (if more numerous), and far fewer positions are available for further hydrogen-bonding (e.g., the fused cube structure only has eight such positions compared with 30 on the dodecahedral cluster). Such clusters are therefore thought (by this author) unlikely to be found in real situations. The collapsed clathrate structure (similar to H3O+(H2O)20 above) for (H2O)21 (with ring structure (20,1)8) is preferred over the convex cluster (with ring structure (20,1)10) in contrast to the preferred convex clathrate structure of H2S.(H2O)20, rationalizing the formation of solid H2S but not H2O clathrates [1114].
The H3O+(H2O)20 cluster, from [72]
Isolated H3O+(H2O)20 clusters with the H3O+ in the central cavity may be distinguished from those with the H3O+ in the surface as they have ten dangling OH groups pointing out from the surface of the distorted dodecahedron rather than 9. Replacement of these water molecules by tert-butanol indicates a 'magic number' stability with up to nine tert-butanol residues (H3O+(H2O)11t-but9), but ten or more residues produce less stable clusters. Thus, the presence of surface H3O+ is established in isolated H3O+(H2O)20 clusters [2290].
The abundance spectra of deuterated heavy water clusters are similar to those of protonated light water clusters, except for a slightly reduced excess stability of the N = 21 cluster relative to the N = 22 cluster [3574]. By applying two-color, infrared-infrared photo-dissociation mass spectrometry to the D3O+(HDO)(D2O)19 isotopologue of the “magic number” protonated water cluster H3O+(H2O)20', it is found that the O-H group can occupy any one of the five spectroscopically distinct sites in the distorted pentagonal dodecahedron cage structure [4129]. The O-H frequency is observed to evolve over relatively long periods of tens of milliseconds in the temperature range (90 to 120 K), while the vibrational signature of the cluster remains intact.
Although there is a preference for the oxonium ion to occupy a cluster surface position in an isolated cluster, this is not likely the case in bulk water. If not ignored in such gas-phase and theoretical studies concerning ionic clusters, this is often understated when results are extrapolated, directly or by inference, to the bulk water scenario.
The low-energy hydroxide ion cluster OH−(H2O)20 behaves differently with the OH− ion centrally placed, accepting four hydrogen bonds and donating one hydrogen bond [1828].
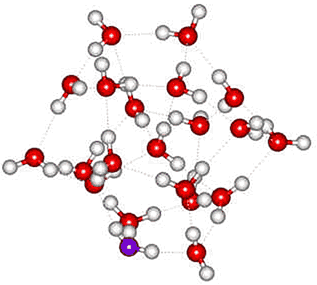
In NH4+(H2O)20, the NH4+ ion sits centrally in the water dodecahedron with all the hydrogen bonds from the central ammonium ion equivalent, helping to explain the apparent increased structural stability of this ion relative to H3O+(H2O)20. In agreement with this, an ab initio molecular dynamics simulation found NH4+ to form four relatively long-lived hydrogen bonds c to tetrahedrally arranged water molecules [136], and such a structure is found to be much more stable than a cluster with an NH3 in the center and an H3O+ on the surface [194]. The exchange of hydrogen bond partners by the central NH4+ may explain its faster than expected rotation [855]. Somewhat surprisingly, tetramethylammonium ions do not form clathrate species in the gas phase with 20 water molecules [3679]; although the number of hydrogen bonds would be maximized, their individual strength is lessened due to their elastic stretch. Other hydrogen-bonding molecules can substitute for water in these clusters (for example, methanol, with its methyl group pointing away from the cluster, H+(H2O)21-n.(CH3OH)n, n =1, 2, 3...) or add to the outside of the cluster (e.g., acetonitrile, H+(H2O)21.(CH3CN)n, n =1, 2, 3... ) [587].
The energies of H2O(H2O)20 clusters with H2O inside an (H2O)20 dodecahedral clathrate cage have been determined [1114]. It was found that the puckered cluster (similar to the top structure of the H3O+(H2O)20 magic ion) was 38 kJ ˣ mol−1 more stable compared with the convex clathrate containing the H2O molecule [1114]. As the puckered structure can undergo rearrangement rapidly, this explains why no crystalline aqueous clathrate structures contain water molecules in their cavities.
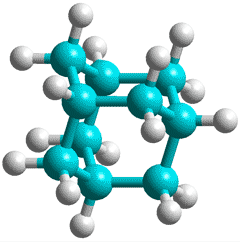
Adamantane, C10H16
Similar magic number water clusters have been found to form around centrally placed Cs+ ions, i.e., Cs+(H2O)20, using vibrational predissociation spectral analysis [2247].
Magic number water clusters (H2O)n have also been observed at n = 49, 51, 53 and 55 for I−, Br−, Cl−, and tetrapropyl-ammonium (TPA+) with their stability attributed to networks of water molecules past the second solvation shell that accommodate these ions through weak water–ion interactions [2812].
A different type of magic cluster may be formed where twelve adamantane molecules surround, in an icosahedral arrangement, twenty water molecules that form a pentagon dodecahedral structure. Each adamantane is placed above the center of a pentagonal face [3393].
[Back to Top ![]() ]
]
a There are many possible hydrogen-bonding arrangements. The one shown has been chosen for comparison to the modeled minimum-energy structure [72]. [Back]
b In the top structure above, a hydrogen bond is shown donating to the oxonium ion (H3O+). This is expected to be ordinarily very weak (or nonexistent) but strengthened in the structure shown due to the possibility of the nuclear delocalization from proton hopping. [Back]
c Note that a single H2O hydrogen-bonded NH4+ ion forms one of the strongest hydrogen bonds known at 92.5 kJ ˣ mol−1 [447]. [Back]
Home | Site Index | Dodecahedral puckering | Clathrates | Ions | LSBU | Top
This page was established in 2000 and last updated by Martin Chaplin on 12 January, 2022