

'I would not put a thief in my mouth to steal my brains'
William Shakespeare, Othello, 1603
Chain of methanol molecules
Methanol, ethanol, and propanol are miscible with water in all proportions to form non-ideal solutions. When water is mixed with alcohol, hydrogen bonds can form between the alcohol molecules, between the water molecules and between water and the alcohol molecules. Whereas water can form four hydrogen bonds (two H-acceptors and two H-donors), alcohols can only form three (two H-acceptors and one H-donor). Some of the H-acceptor sites in any aqueous-alcoholic mixture must be unfulfilled. In mixed hydrogen bonding, proton acceptor from the water (RHO····H-O-H) is more favorable than that of proton donor from the alcohol (RO-H····OH2). Low molecular weight alcohols form zigzag chains of molecules connected by single donor and acceptor hydrogen bonds (see above left) [2700]. Pure methanol forms hydrogen-bonded networks containing hexamers and octamers [4273], and such clusters are expected in aqueous-alcoholic solutions.
Ethanol g
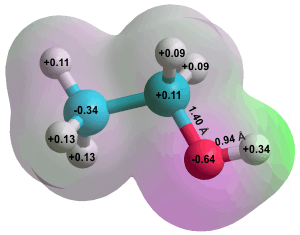
Aqueous alcoholic solutions e have been reviewed [2354]. Low relative molecular mass (molecular weight) alcohols freely mix with water and are widely used as solvents and, in the case of ethanol, in alcoholic drinks. Although mixing freely, the resulting liquid is not homogeneous but consists of water and alcoholic and mixed water/alcoholic clusters. Methanol and ethanol show negative excess entropies of mixing [2718]. On adding the water, the liquid microjet X-ray absorption spectra show significantly enhanced hydrogen-bonding of the methanol and ethanol hydroxyl groups resulting in the reduction in entropy and negative enthalpy of mixing due to the more significant clustering. As we add ethanol to water, the water hydrogen bonds strengthen with the water forming stiff clathrate cages around the ethanol molecules. However, as the volume ratio increases beyond 20%, a phase transition occurs where the water goes from its cage-like structure to forming hydrogen-bonded links between the ethanol molecules [3033]. The competition between hydrophilic and hydrophobic interactions in methanol/water mixtures is different between freezing and warm temperatures, with the physical properties also determined by the temperature [3109]. In the supercooled regime, the hydrogen-bonding interactions have a lifetime long enough to sustain a stable water network. However, increasing the temperature destroys these water clusters progressively. In addition, at temperatures higher than about 265 K, the hydrophobicity gradually increases to dominate the physical properties of the solutions [3702]. Using molecular dynamics simulations and a core-softened potential approach, it has been shown that methanol can suppress the liquid-liquid phase transition in water [4222].
Analysis of the clustering in ethanolic solutions depends slightly on the definition of the hydrogen bond used, with the looser definitions giving the least amount of free molecules [3071]. The solutions are micro-heterogeneous, with
the alcohols tending to form linear but zigzagging, hydrogen-bonded chains
with each other (with increasing effect with the alcohol hydrophobicity).
At low alcohol contents, the water molecules form tetrahedral clusters [776], c mostly excluding the alcohol molecules (although at higher temperatures, mixed clusters predominate [1569]). Aliphatic alcohols create one strong hydrogen bond to water, about 75% of the strength of a water-water hydrogen bond, but further hydrogen-bonding to the two further possible sites is much weaker [2355]. There appear to be few hydrophobic contacts within aqueous solutions of alcohols ranging from methanol to tertiary butyl alcohol, as the interactions between their small hydrophobic groups are weaker than any thermal energy fluctuations. These small hydrophobic groups are surrounded by relatively strong water clusters, removing the driving force for hydrophobic interactions [2432].
Vapor-liquid equilibrium diagram for 1-propanol-water
![Vapor-liquid equilibrium diagram for 1-propanol-water Vapor-liquid equilibrium diagram for 1-propanol-water; also the hydrogen bonding weakening [2331]](images/azeotrope.gif)
Alcoholic solutions may contain several distinct liquid phases dependent on solutes, temperature, and pressure [1297] with greater segregation at higher pressures [2769] and, in the case of glycerol, at lower temperatures [2770]. In contrast, simulations indicate that water molecules form long chains in freezing ethanolic solutions with lower segregation [3087].
In line with their hydrophobic nature, the alcohols preferentially occupy surface sites, up to maximum values equivalent to about a mono-layer, and lower the surface tension [777]. At higher concentrations (molar alcohol:water > 0.1), clear micro-aggregation occurs [777]. Binary mixtures of low molecular mass alcohols (e.g., ethanol, 1-propanol, and 1-butanol but not methanol [2452]) show azeotropy a with water, caused by the changes of the evaporation properties of the solution with composition. The mole fraction of ethanol, 1-propanol, and 2-propanol in their azeotropic mixtures are 89%, 43%, and 68%, respectively.
A comparison of the vibrational, reorientational, and hydrogen bond dynamics of ethanol in water and in hexane has been made [4141]. Ethanol is fully miscible in both solvents and forms micelle-like domains in water but cyclic clusters, resembling loops, in hexane.
The effect of increasing mole fraction is shown for 1-propanol (above left [1746]). At low 1-propanol composition, hydrogen-bonded water clustering excludes the alcohol so that the alcohol evaporates more readily. At high alcohol content, clustering of the alcohol molecules due to inter-alkyl van der Waals forces plus hydrogen-bonding interactions exclude the water molecules such that the water evaporates preferentially [1746]. Similarly, low methanol concentrations do not interfere with the water structuring, whereas high methanol concentrations destroy the structure of water as they leave alcohol-water aggregates [3119] and long-range correlations [4312]. In aqueous methanol, ethanol, and 1-propanol solutions, the more hydrophobic methyl chains raise the intensity ratio of the 3430 cm−1 sub-band to the 3220 cm−1 peak, indicating that the single donor-acceptor water molecules form at the interface between these hydrophobic areas and water. Mixtures of water and ethanol exhibit a steep increase in the surface ethanol molecules up to a 0.1 mole fraction with increased ordering [4277].
Partial molar volumes of water and ethanol, from [2116] and [2885]
![partial molar volumes of water and ethanol in their mixture, from [2116] and [2885] partial molar volumes of water and ethanol in their mixture, from [2116] and [2885]](images/etoh-h2o.gif)
The hydrogen-bonding strength may be assessed from the v2+v3 overtone at about 5210 cm−1 [2331]. High water content weakens the average strength of hydrogen bonding in the mixture as individual water–water interactions are then weaker than those between the alcohol and water [1746].
The partial molar volumes of water and the alcohols usually present a complex picture (see right) where the total volumes are reduced compared with the sum of the individual volumes. This complexity is due to the balance of the water-water and solute-water interactions [2420], with the net loss of strong hydrogen-bonding. The speed of sound similarly varies with the mole fraction (see right), indicating a stiffening of the structure at low (≈ 0.1) mole fractions [2885], in line with the Raman results [3033]. The self-diffusion of water in ethanol-water mixtures has been reported [3594]. The thermal conductivity of ethanol-water solutions behaves similarly to the speed of sound but with a maximum at about 0.025-mole fraction of ethanol [4079]. In the very diluted region (xethanol< 0.025), water shows greater tetrahedral order and stiffer hydrogen-bonding, whereas when 0.025 < xethanol< 0.1, the hydration shells coalesce, and the alkyl chains begin to associate, promoting increasing hydrogen-bonding between water and the -OH group of the alcohol. Above 0.1 mole fraction, hydrogen-bonding between ethanol molecules increases, and the thermal conductivity reduces further. The interactions with methanol has also been noted [4160].
The experimental Raman spectra and molecular dynamics tetrahedrality of water molecules in the hydration shells of short-chain alcohols have been determined [3404]. These undergo a crossover above 100 °C (at 30 MPa) to less tetrahedral structures than pure water.
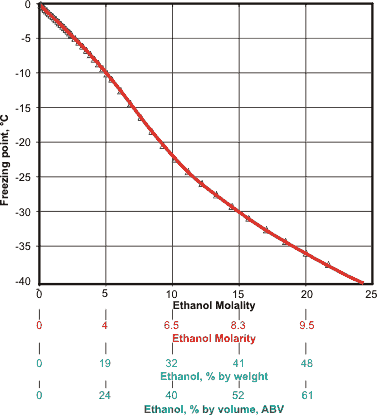
The freezing point of ethanol-water
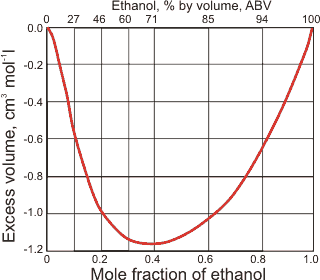
The excess volume of mixtures of ethanol and water is always negative, showing that any combination of water and ethanol shrinks in volume (see below right) due to the attraction between water and ethanol.
The excess volume of ethanol-water
The freezing point of aqueous ethanol is displayed on the left, showing that typical spirits (40% ABV) remain liquid in a domestic freezer.
The water activity of aqueous ethanol solutions, from [2920]
![The water activity of aqueous ethanol solutions, from [2920] The water activity of aqueous ethanol solutions, from [2920]](images/etohaw.gif)
The water activity of ethanolic solutions is shown left [2920].
The Alcohol percentage By Volume (ABV) data allows comparison between alcoholic drinks. b Typical values are,
| Drink e | ABV | Drink | ABV |
| Lager | 4 - 5 | Sherry | 18 - 20 |
| Cider | 3.5 - 5 | Port | 19 - 20 |
| White wine | 11 - 13 | Gin | 37 - 43 |
| Champagne | 11.5 - 12.5 | Rum | 37 - 50 |
Dmitri Mendeleev (of the Periodic Table fame) suggested that vodka should be about 38% ethyl alcohol by volume (5 H2O : 1 ethyl alcohol) in 1893. Russian law followed in 1894, stating that all vodka had to be produced at 40% alcohol by volume.
The rotational and translational dynamics of neutral (CO, NO) and charged (CN−) diatomic species have been determined in simulations of water-ethanol mixtures [4007]. The opposite behavior is found between the neutral and charged species. The self-diffusion constants of both CO and NO initially decrease and then increase with the increasing ethanol composition after attaining a minimum at 10%. However, CN−'s self-diffusion constant has about the same value as these two in water but continuously reduces with increasing ethanol composition.
Protonated alcohols present a much simpler structure to protonated water, as they consist of simple chains and rings with no three-dimensional aspects [3223].
Methanol/water dynamics have been investigated using quasielastic neutron scattering employing an isotopic substitution [4407]. This showed that nanoscopic association among unlike molecular species caused the viscosity of the mixtures at intermediate concentrations to be higher than that of the pure components. The structural details, viscosity trends, and dynamic phenomena in aqueous t-butanol solutions have been investigated using small- and wide-angle x-ray scattering (SWAXS) method and molecular dynamics simulations [3824]. Four composition ranges with qualitatively different structures and viscosity trends were revealed, involving five t-butanol -water hydrogen-bonding lifetimes. Both hydrogen bonds and hydrophobic effects were found to be important in the formation of the t-butanol aggregates. A molecular dynamics study using graph theory has revealed that the alcohol aggregates in fully miscible aqueous methanol and ethanol mix well with water and disrupt its hydrogen-bond networks. In contrast, the networks of n-butanol aggregates do not mix well with water and little alter the remaining water structure [4104]. This explains why n-butanol is not completely miscible in water, forming two liquid phases between ~0.02 and 0.5 alcohol mole fractions. More hydrophobic alcohols, such as t-butanol, 2-butyl alcohol, and 2-butoxyethanol, behave similarly to methane in solution [4107] and encourage clathrate structures formation [4108], although these are much more fluid than the solid hydrates. f At higher concentrations, these more hydrophobic alcohols tend to disrupt water structure and decrease the tetrahedrality of the local water.
The butanol isomers , n-butanol, sec-butanol (s-butanol), isobutanol (i-butanol) and tert-butanol (t-butanol) have distinct
liquid–liquid phase diagrams, with t-butanol fully miscible in water, whilst the otherss are only partially miscible under ambient
conditions [4298]. The small tert-butanol aggregates causes significant disruption of the water H-bond network, forming a homogeneous solution, whereas the other butanols form large aggregates excluding ther water.
Aqueous alcohols are part of many aqueous co-solvent mixtures of small polar molecules such as amides, alcohols, dimethylsulfoxide, or ions in water that seem fully miscible but are heterogeneous on the nanometer scale [4074]. Hydrogen-bond networks are mostly preserved in the water-rich regions but are composition-dependent at lower water contents. The dynamics of the mixture span the timescales from picoseconds and nanoseconds, for hydrogen bonding timescales, to milliseconds and seconds for bulk reorganization.
[Back to Top ![]() ]
]
First described by Lord Kelvin's brother in 1855, d tears can be seen as a ring of clear liquid near the top of a glass of alcoholic drink, from which droplets continuously form and drop back into the beverage (the Gibbs–Marangoni effect). It is most evident in strong wines and spirits. As the alcohol has a lower surface tension than water, a solution of increased alcohol content rises up the glass [3042]. Preferential evaporation of the alcohol then increases the water content in this thin layer causing its surface tension to rise and for the liquid to drop back into the drink.
[Back to Top ![]() ]
]
a An azeotropic mixture is one that has the same composition in the vapor and liquid phases and therefore cannot be separated by distillation. [Back]
b The ABV (alcohol by volume) is expressed as a percentage, often simply as % vol. It is defined as the number of milliliters (mL) of pure ethanol present in 100 mL of solution at 20 °C (68 °F). It is not equal to the % by volume (% v/v, or % volume fraction). For example, a 40 % ABV vodka contains 40 mL of ethanol in each 100 mL of vodka, but the vodka comprises more than 60 mL of water in every 100 mL of vodka.
In the United States, the amount of alcohol is specified as alcohol proof, which is twice the ABV number. [Back]
c Water may form linear chains with other solutes, such as dimethyl sulfoxide (DMSO) [2481, 3348]. [Back]
d J. Thomson, On certain curious motions observable at the surfaces of wine and other alcoholic liquors," Philosophical Magazine, 10 (1855) 330-333.. [Back]
e The British National Health Service had recommended that men and women should not regularly drink more than 3-4 units or 2-3 units of alcohol a day respectively. This recommendation has now been reduced to 14 units a week, spread evenly over three or more days, for both men and women. One unit equals 10 mL (≈ 8 g) of pure ethanol, which is around the amount of alcohol the average adult can process in an hour. The units in any drink may be calculated by multiplying the total volume of a drink (in mL) by its ABV (see b above) and dividing the result by 1,000; thus, a 'typical' bottle of wine (75 cL, 12 % vol ABV) contains 750 ˣ 12/1000 = 9 units which are more than twice that recommended for a couple in a day. A pint of beer (ABV 4 %) contains 2.3 units (UK pint), 1.9 units (US pint). When trying to reduce the amount of alcohol driunk, it is a good idea to have several alcohol-free days each week. When pregnant or trying to become pregnant, it is safest to avoid alcohol altogether. Drinking excessive alcohol can affect the quality of sperm.
Driving a car (or other vehicles) is significantly affected by drinking just one unit of alcohol. A person is probably legally drunk after drinking just five units of alcohol. Drunk-in-charge driving (driving under the influence of alcohol, DUI) is judged by the amount of alcohol in the blood (mg/100 mL) or breath (µg/100 mL). The limits vary between different countries. [Back]
f The debate is not over, as other workers do not find increased tetrahedrality [4109]. [Back]
g The molecular structures on this page were calculated in the gas phase, using the Restricted Hartree-Fock wave function (RHF) using the 6-31G** basis set. This is in line with other calculated structure factors given in this website (e.g., the values for the water molecule and its clusters). [Back]
[Back to Top ![]() ]
]
Home | Site Index | Solubility of nonpolar gases | Hydrophobic hydration | Hydrocolloids | Polysaccharide hydration | Protein hydration | Nucleic acid hydration | Aqueous biphasic systems | Gas-liquid interface and nanobubbles | LSBU | Top
This page was established in 2015 and last updated by Martin Chaplin on 13 July, 2022