
Singlet molecular oxygen, 1O2
Molecular oxygen is very reactive but necessary for life as we know it.
![]() The reaction of water with ionizing radiation
The reaction of water with ionizing radiation
Animal life depends on the solubility of oxygen in water
Oxygen (O2) is commonly found but very reactive. All young worlds possess little molecular oxygen, and we only have a rich oxygen atmosphere due to photosynthesis.
6 CO2 + 12 H2O* + sunlight (2897 kJ) ![]() C6H12O6 + 6O*2+ 6 H2O
C6H12O6 + 6O*2+ 6 H2O
balanced by the oxidation of the foodstuffs (glucose in this case) to give organisms the energy for life's processes
C6H12O6 + 6O2 ![]() 6CO2 + 6H2O + life's energy
6CO2 + 6H2O + life's energy
Dissolved oxygen in water can be determineda by a redox electrode with an oxygen-permeable membrane placed over the electrodes. The membrane only allows molecular oxygen to pass from the solution.
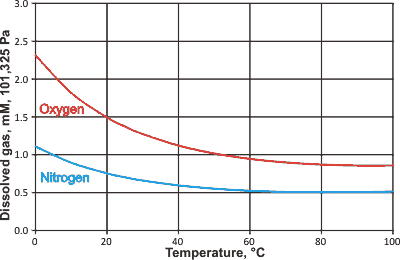
Oxygen is a poorly soluble hydrophobic molecule [1020] that exists in (H2O)20 clathrate cages on dissolution [3570]. The solution is accompanied by a reduction in enthalpy and entropy of the solution. The greater equilibrium solubility of O2 over N2 (see below right and gas solubility), despite its lesser clathrate forming ability [1168], has been proposed due to its formation of weak hydrogen bonds to water [1168]. The formation of O=O···H-OH hydrogen bonds may be seen as the first stage in the natural low-level formation of oxygen redox products (for example, H2O2) in water. As the ratio of O2/N2 solubilities has a maximum at 290 K, there is an indication that partial clathrate cages may be responsible for the polarization that encourages the hydrogen bond formation. At surfaces and within confined spaces at medium relative humidities (RH, 55 %), the solubility of oxygen (O2) increases up to two-fold [3437].
Equilibrium solubility of O2 and N2 with temperature
from [ IAPWS ]
Water stores and transmits information concerning solutes using its hydrogen-bonded network. Changes to this clustering network brought about by gaseous solutes may take some time to re-equilibrate. It has been shown that a high magnetic field has an insignificant effect on the equilibrium content of dissolved oxygen (< 0.3 mM at 20 °C under atmospheric conditions) but does significantly enhance its dissolution rate [176]. There is one report that magnetically treated water (also from the same laboratory, electromagnetically treated water) retains a significantly changed effect on fungal spore germination for at least 24 hours [174]; however other parameters (for example, reduced dissolved oxygen levels) may be responsible for such effects. Mechanically-induced hydrogen bond breakage, caused by shaking, has been reported to last for weeks [336].
High electric fields (E ≈ 109 V m−1) reduce water's permittivity [616], which will increase the solubility of gases. Water may be supersaturated with oxygen (≈ 3-6 mM; equivalent to less than a breath of air in each liter of supersaturated water) under pressure. It should be noted that left by itself, degassed water may take days to re-equilibrate with all atmospheric gases except for CO2 that dissolves much faster. As even small amounts of dissolved gases are reported to have relatively large effects on water structuring [560], it is not unreasonable to suppose that artificially induced metastable conditions with higher gas content may last for some time. Drinking of oxygenated water does give a transient moderate increase in serum ascorbyl radicals (with unknown consequences), an effect that disappears with regular consumption [422]. However, it will not significantly add to the body's oxygen intake and has no apparent harmful or health-promoting effects [772].
Triplet oxygen may convert to singlet dioxygen under near-infrared irradiation in solution [2275], despite this transition being 'forbidden' in isolated molecules
λ = 1264 nm
3∑g 3O2 ![]() 1O2 1Δg
1O2 1Δg
followed by,
slow
1O2 + H2O ![]() HO2· + ·OH
HO2· + ·OH
Production of singlet oxygen (1O2;1Δg+, electrons paired in their π-antibonding molecular orbitals, compare 3O2, normal triplet oxygen, 3Σg−, where two electrons are in equivalent but separate π-antibonding orbitals with the same unpaired spin) during processing may cause the dissolved peroxide concentration to increase via the water-catalyzed H2O-oxidation reaction;
x.1O2+2H2O ![]() (1 –x ).3O2+2H2O2 [1199]
(1 –x ).3O2+2H2O2 [1199]
with possible consequential pharmacological effects. Interestingly singlet oxygen participates in antibody-catalyzed water oxidation similarly producing triplet oxygen and hydrogen peroxide [624]. However, as the lifetime of the singlet oxygen is expected to be in the μs range when dissolved upwards towards 45 min in the (low pressure) gas phase, singlet oxygen molecules are not expected to remain in the processed bottled water.
[Back to Top ![]() ]
]
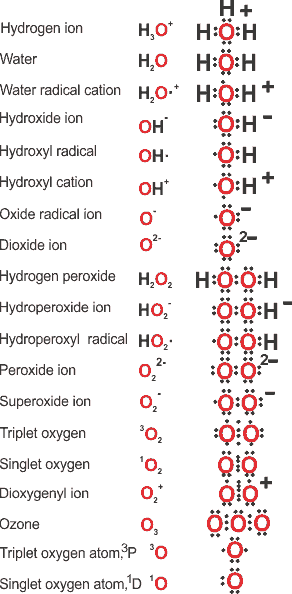
Lewis electron configurations
Free radicals (for example, hydroxyl radicals) and free
(hydrated) electrons can be introduced into water by techniques
such as electrochemistry, ultrasonics, nanobubble production [2637], by direct water photolysis, by ultraviolet radiation
(≈ 150 nm, [497], 253.7 nm, [3102] disinfection radiation of ice at very low temperatures [3678], or simply by agitation. Radicals are reactive species. They possess a singly occupied molecular orbital (SOMO) SOMO. This half-filled orbital has both the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) character, with a zero HOMO-LUMO gap. Such radical processes are catalyzed by the trace amounts (ppb) of Fe2+/Fe3+ often present in purified water. These reactive oxygen species (ROS) occur naturally in aerobic life at low physiological levels and have a central role in redox signaling [3986]. The ratio of Henry’s law constants (![]() in m3 ˣ atm ˣ mol−1) for O2, O3, •OH, HO2•, and H2O2 are 1.2 ˣ 10−5 , 1 ˣ 10−4 , 0.38, 6.8, and 910 respectively [3406]. High levels of these oxygen species are, however, may lead to molecular damage.
in m3 ˣ atm ˣ mol−1) for O2, O3, •OH, HO2•, and H2O2 are 1.2 ˣ 10−5 , 1 ˣ 10−4 , 0.38, 6.8, and 910 respectively [3406]. High levels of these oxygen species are, however, may lead to molecular damage.
Water can break down in several ways to form reactive products,
H2O ![]() e−(aq) + H2O·++ H3O+ + ·OH + OH− + OH+ + H· +
e−(aq) + H2O·++ H3O+ + ·OH + OH− + OH+ + H· +
H2O2 + HO2· + H2 + O2·− + 1O2
The radical cation of water (H2O·+) is often formed first from water's ionization but has a very short lifetime (≪1 ps). H2O·+ is the acid form of the hydroxyl radical ·OH [3472] and the most potent oxidizing agent in liquid water [3848]. Due to its positively charged hydrogen atoms (~+0.5 e−), its bond lengths are 4% longer than those of H2O,and its H-O-H bond angle has expanded to almost 113° (using the 6-31G** basis set). It usually rapidly (< 1 ps) reacts as a strong acid by proton transfer to form the ·OH radical.
H2O·+ + H2O ![]() ·OH + H3O+
·OH + H3O+
UV light can break down H2O to give ·OH + ·H giving rise to many other species [4473], e.g.,
H2O + hv185nm ![]() •OH + H•
•OH + H•
H2O + H• ![]() H3O+ + e−aq pKa = 9.7
H3O+ + e−aq pKa = 9.7
2 •OH ![]() H2O2
H2O2
Radicals can initiate chain reactions involving cascades of reactions; for example, a single ·OH hydroxyl radical may form 34 peroxide molecules [3102]. Such hydroxyl radicals may also be created by 190-300 nm radiation exciting an electron from the hydroxide ion [3022]. Water may also be split by electrolysis or mechanical methods, such as ultrasonics or stirring with a catalyst [739], to give H2 and O2, plus some associated free radicals such as the highly reactive hydroxyl radical. In particular, low concentrations of hydrogen peroxide (H2O2) may be produced from water (H2O) by any process that moves clusters of water relative to each other such as mechanical vibration and stirring [1066].
Hydrogen peroxide
(H2O)n(H2O ![]() H-OH
H-OH![]() OH2)(H2O)m
OH2)(H2O)m ![]()
(H2O)n(H2O + H· + ·OH + OH2)(H2O)m
2 ·OH ![]() H2O2
H2O2
without the need for molecular oxygen but increased by it [1066], for example,
(normal triplet oxygen) 3O2 + ·H ![]() HO2·
HO2·
HO2·+ ·H ![]() H2O2
H2O2
HO2·+HO2· ![]() H2O2 + 1O2
H2O2 + 1O2
(highly reactive singlet oxygen)
1O2 + 2H2O ![]() 2H2O2
2H2O2
H2O2 torsional energies
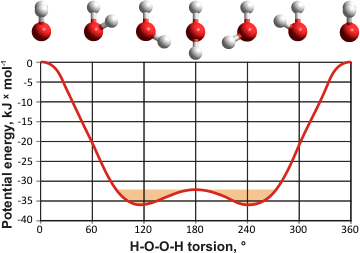
The hydrogen peroxide structure (see above right has been calculated using the Restricted Hartree-Fock wave function (RHF) and the 6-31G** basis set. Torsional rotation around the central O-O bond gives two positions of minimum energy (116.26° and 243.74°, see left) and a high rotational barrier due to repulsion between the lone pairs of the oxygen atoms (cis, at 0°). These two minima are connected by a low-energy saddle-point (trans, at 180°) of approximately thermal energy (shown orange). The H2O2 molecule rapidly changes its torsional angle between these minima but does not fully rotate.
H2O2 is a weak acid with pKa = 11.65 (25 °C). A second pKa has not been found but is likely to be > 22. A concentrated solution (70% w/w, 26.4 M, mole fraction = 0.5527) has a pH below one, a boiling point of 126 °C, and a melting point of -40 °C. The pure liquid has a boiling point of 150 °C, and a melting point of 0 °C. It can form a hydrate, H2O2.2H2O below -55 °C. The H2O2 hydrogen atoms carry a charge of +0.365, similar to those on water, with the O–H bonds of similar length to those in H2O. The minimum energy structure gives a slightly greater molecular dipole moment (2.26 D) than has water (1.85 D). H2O2 is an oxidizing agent more potent than chlorine but weaker than ozone,
H2O2 + MH2 ![]() 2H2O + M
2H2O + M
where M represents part of the oxidized material. H2O2 can also be a reducing agent (for example, it reduces sodium hypochlorite in the preparation of oxygen),
NaOCl + H2O2 ![]() NaCl + O2+ H2O
NaCl + O2+ H2O
Hydrogen peroxide is unstable and slowly breaks down to give water and triplet oxygen (normal oxygen),
2H2O2 ![]() 2H2O + 3O2 ΔG° = -77 kJ ˣ mol−1
2H2O + 3O2 ΔG° = -77 kJ ˣ mol−1
This decomposition can be rapidly accelerated by catalysts such as platinum metal and the enzyme catalase. Other materials (e.g., the commonly present Fe2+, the Fenton reaction. see below) can catalyze its breakdown to free radicals such as hydroxyl OH• and hydroperoxyl HOO•. The Fenton reagent (a mixture of hydrogen peroxide and ferrous iron) is one of the most effective methods for the oxidation of organic pollutants. The Fenton reaction may be used as a feed pre-treatment in the production of effluent more easily handled by membrane distillation [3810]. Hydrogen peroxide is more stable when at slightly acid pH and lower temperatures.
The Fenton reaction, from [3515]
![The Fenton reaction, from [3515] The Fenton reaction, from [3515]](images/fenton..gif)
It is a chain reaction, with the chain initiation step and first chain reaction step,
Fe2+ + H2O2 → Fe3+ + OH− + OH•
further chain reaction steps
OH• + H2O2 → O2•− + H3O+
Fe3+ + O2•− → Fe2+ + O2
and chain termination steps
Fe2+ + OH• → Fe3+ + OH−
Fe2+ + O2•− + 2 H3O+→ Fe3+ + H2O2 + 2 H2O
RH + OH• → R• + H2O
Hydrogen peroxide decomposition mechanisms in the absence and presence of iron ions (varying between FeI, FeII, and FeIII) in aqueous solution, which contain no OH• formation, have been theoretically determined [4374]. The reaction pathway in the absence of iron ions is given as,
2 H2O2 → H2O + HOO(H)O → 2 H2O + O2
where H-O-O(H)-O is a hydrated molecule, different from but isomeric with H-O-O-O-H. This work concluded that H2O2 decomposition in the presence of the iron ions is driven by electron transfer to an iron ion hydration complex and proceeded by hydrogen transfers in the hydrogen bond network around H2O2.
A bicarbonate activated hydrogen peroxide (BAP) system [4303] catalyzes peroxide breakdown
HCO3− + H2O2 → HOCO3−+ H2O
followed by oxidative chain reactions such as,
HOCO3− → •CO3− + HO•
•CO3− + H2O2 → HCO3−+ HO2•
Hydrogen peroxide may also break down under UV irradiation to form strongly oxidizing hydroxyl (HO·) and hydroperoxyl (HO2·) radicals.
λ = 250≈ 420 nm
hν + H2O2 ![]() 2 OH•
2 OH•
OH• + H2O2 ![]() HO2• + H2O
HO2• + H2O
Hydrated superoxide anion, O2·−
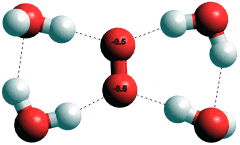
The hydroperoxyl radical (HO2•) is a weak acid (pKa = 4.8) and ionizes to give the superoxide radical anion (O2•−). The superoxide radical anion (O2•−) hydrates in a planar manner (see right) to H-O protons from four water molecules [2007]. It decays by reaction with its conjugate acid HO2·,
O2•−+ HO2• + H2O ![]() H2O2 + O2 + OH−
H2O2 + O2 + OH−
in water at a rate constant of about 108 M−1 ˣ s−1, which gives a half -ife of about a second, when both O2•− and HO2• are at micromolar concentration.
·OH (H2O), top H-bonded; bottom hemi-bonded
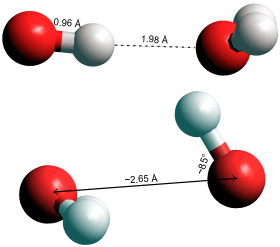
The hydroxyl radical, OH•, is highly reactive if short-lived (< 1 ns in biological systems) [3922]. It forms naturally in the atmosphere (from ultraviolet rays, ozone, and water vapor), where it lasts about one second before reacting [3477]; being the most common oxidant in the troposphere. It can also form at anode surfaces [3722]:
H2O ![]() H+ + OH• + e−
H+ + OH• + e−
The hydroxyl radical can form hydrogen bonds to water (see left above) or hemibonds to water (a two-center, three electron bond, see left, lower diagram) [4170]. The proportions of these species have not yet been established.
Hydrated hydrogen peroxide, H2O2
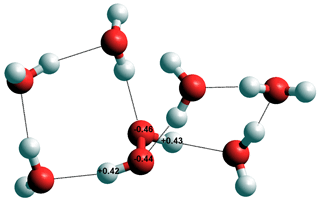
OH• is also a highly potent oxidizing agent where it picks up an electron to become the hydroxyl ion (OH−). It preferably hydrogen-bond to water by donating its H-atom, rather than accepting H-bonds from water (calculated using the Restricted Hartree-Fock wave function (RHF) using the 6-31G** basis set). The hydrogen bond is shorter and stronger than that of the water dimer. The changes in the ·OH stretch with temperature and density have been investigated using molecular dynamics simulation [3470]. The hydroxyl radical, ·OH, is the atmosphere’s most important detergent, breaking down many gases (but not CO2) so counteracting 'global warming'. The hydroxyl radical, OH•, removes hydrogen atoms from organic molecules, leaving the organic radicals to react further,
OH• + RH ![]() H2O + R•
H2O + R•
R• + O2 ![]() RO2•
RO2•
There is an experimental and theoretical study of the gas-phase reactions between the singlet oxygen atom O(1D) with H2O (and D2O) [4375] that is relevant to the chemistry of the Earth's atmosphere,
O(1D) + H2O ![]() 2 OH•
2 OH• ![]() O(3P) + H2O
O(3P) + H2O
Other more exotic compounds of oxygen and hydrogen have been examined; for example, H2O3 (H-O-O-O-H, [2498] made from H2O2+ O3, H2O4 [2500] the (HO2)2 dimer, and planar cis- and trans-hydridotrioxygen (HO3•) formed from •OH and O2 [2499].
Ozone, O3
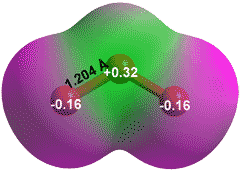
Ozone (O3, see right) is a symmetrical singlet di-radical. As a gas, it has a pale blue color turning dark blue as a liquid. It has a distinctive sharp electrical-related odor, a boiling point of 161.3 K, melting point of 80.7 K, and critical point at -12.0 °C and 5.5 MPa. It is a powerful oxidant, much more oxidizing than oxygen, toxic, reactive, corrosive, and treated as an air pollutant. It is a bent (119°) polar molecule with a dipole moment of 0.53 D. Ozone is produced naturally by the action of lightning or high-energy photons (160 - 240 nm) on oxygen in the air,
slow
O2 + photon ![]() O + O
O + O
fast
O + O2 ![]() O3
O3
and the steady-state reversal slow
O3 + O ![]() O2 + O2
O2 + O2
Ozone in the atmosphere
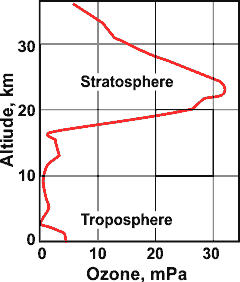
As seen right, the greatest concentration of ozone in the atmosphere is within the stratosphere and it usefully blocks ultra-violet light (200 - 280 nm) from reaching the surface. However, low-level ozone in the troposphere is an atmospheric pollutant produced partly from (not in) reactions of car exhausts in densely-populated areas and is a critical constituent in smogs. It has a half-life of about 22 days.
Ozone is 14 ˣ more soluble in aqueous solutions than oxygen. It is made by electrolysis [3461], chemical radiation, and corona discharge (electrical discharge) of air and is used to sterilize medical instruments and to detoxify (oxidize) wastewater. It easily adds across carbon double bond leading to (oxidative) breakup of the structure. It may also produce hydrogen peroxide at air-water interfaces [4370]
O3 (g) ![]() O3 (aq)
O3 (aq)
O3 (aq) + H2O ![]() O2 (aq) + H2O2 (aq)
O2 (aq) + H2O2 (aq)
Ozone is unstable, and liquid ozone may detonate. It may produce strongly oxidative radicals (e.g., •OH) on decomposition, particularly in dilute alkaline solution.
2O3 ![]() 3O2
3O2
O3 + OH− ![]() HO2− + O2
HO2− + O2
O3 + HO2− ![]() •OH + O2•− + O2
•OH + O2•− + O2
[Back to Top ![]() ]
]
Initial processes in the decomposition of water by ionizing radiation.
from [3413]
![Initial processes in the decomposition of water by ionizing radiation from [3413] Initial processes in the decomposition of water by ionizing radiation from [3413]](images/radiatedh2o.gif)
Many activated species may be produced from the action of ionizing radiation (such as 1 MeV electrons) on water (see right, [3413]). The initial excitations and ionizations occur over femtoseconds, whereas the hydration processes take picoseconds to nanoseconds, with the other processes in between. The products rapidly inter-react and react with other molecules present and their properties are further discussed above.
At low temperatures (<100 °C), hydrogen peroxide is also formed [3918].
·OH + ·OH ![]() H2O2
H2O2
H2O.O2·+
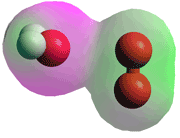
Between altitudes of 80-100 km O2·+ and NO+ions are the dominant cationic species.
O2·+ ions react with water molecules to form H2O.O2·+ species (see figure left) [4020].. At lower altitudes, this further reacts with more water molecules to form hydrogen ions.
[Back to Top ![]() ]
]
Oxidation state (Frost) diagram for H2O /O2 at pH 7,
from [3334].
![Oxidation state (Frost) diagram for O2, from [3334] Oxidation state (Frost) diagram for O2, from [3334]](images/o2red.gif)
The redox reactions of water and oxygen are shown in a Frost diagram (see right) [3334]. It shows nE0 plotted against the oxidation number n, where E0 is the standard reduction potential for the couple, and n is the number of electrons transferred in the conversion. The vertical axis shows the reaction free energy as nE0 = -ΔG/F, so the higher the potential, the more reactive the material (·OH is the most potent oxidant and rapidly and destructively reacts with biomolecules). It also reacts with O2 to form HO3 [3630]. The standard oxidation potentials are the negative slopes of the lines joining any two states that form a redox couple.
The long red line represents,
½O2 + 2H3O++ 2e− ![]() 3H2O E°' = +0.815 V
3H2O E°' = +0.815 V
The lighter blue lines represent,
H2O2 + e− + H3O+ ![]() ·OH + 2H2O E°' = +0.39 V
·OH + 2H2O E°' = +0.39 V
O2·−+ e− + 2H3O+ ![]() H2O2 + 2H2O E°' = +0.88 V
H2O2 + 2H2O E°' = +0.88 V
The light purple line represents
3O2 + 2e− + 2H3O+ ![]() H2O2 + 2H2O E°' = +0.36 V
H2O2 + 2H2O E°' = +0.36 V
The orange line represents
3O2 + e− ![]() O2·− E°' = -0.18 V
O2·− E°' = -0.18 V
The magenta line represents
1O2 + e− ![]() O2·− E°' = +0.81 V
O2·− E°' = +0.81 V
and the long purple line represents,
·OH + e− + H3O+ ![]() 2H2O E°' = +2.31 V
2H2O E°' = +2.31 V
As O2·− lies above the line connecting H2O2 and 3O2, it can act as both oxidant and reductant in the dismutase reaction, rather than the damaging reactions of ·OH
O2·−+ O2·− + 2H3O+ ![]() O2 + H2O2 + 2H2O
O2 + H2O2 + 2H2O
Oxygen redox, from [4055]
![Oxygen redox, from [4055] Oxygen redox, from [4055]](images/oredox.gif)
See also water electrolysis.
[Back to Top ![]() ]
]
a Guide for Dissolved Oxygen Measurements, Mettler-Toledo GmbH (2018) 30519845. [Back]
Home | Site Index | Water and health | LSBU | Top
This page was established in 2001 and last updated by Martin Chaplin on 22 July, 2022