
Silicic acid, Si(OH)4
4.gif)
The chemistry of the silicates involves the strong attraction of silicon to oxygen and their tetrahedral nature.
![]() Orthosilicic acid
Orthosilicic acid
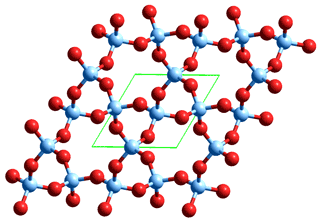
![]() Silica interfaces
Silica interfaces
Silicon (Si) has atomic number 14 with electrons 1s22s22p63s23p23d0. It is a tetravalent metalloid (mostly as SiIV) with distinct properties different from the nonmetal carbon above it in the Periodic Table and the metals tin and lead below it. Si is relatively (to carbon) unreactive and (unlike carbon) does not usually form Si-Si single (SiIII), double, or triple bonds. Si has a high chemical affinity for oxygen. Tetrahedral coordination is a major structural motif in silicon chemistry, just as it is for carbon chemistry. However, the chemistry of silicon shows significant differences from that of carbon, with its electronegativity (1.90) much less than carbon (2.55) as its valence electrons are further from the nucleus and hence experience smaller electrostatic forces of attraction to the nucleus. Silicon atoms are usually joined through oxygen atoms (as Si-O-Si), with silica (SiO2) and silicate (SiO4-x(4-x)−) materials are the most abundant materials in the earth's crust. The combination of their rigid tetrahedral structures is very varied but based on a tetrahedral arrangement of silicon atoms covalently connected by oxygen atoms; each silicon atom is bound to four oxygen atoms. Each oxygen atom is bound to two silicon atoms (see below right).
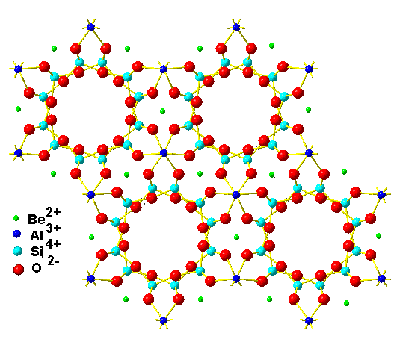
The structure of the silicate beryl
Silicon dioxide (SiO2, the most abundant chemical material in the earth's crust although rarely found in a pure state, see example right), also known as silica, has several different crystalline forms. An example is α-quartz, consisting of SiO2 tetrahedra with Si at the center and connected at their corners, with each oxygen atom linked to two silicon atoms. Silica forms amorphous glasses where the tetrahedral silica elements bind together seemingly randomly. It is particularly reactive with HF.
Adding water to silica may break its structure by replacing Si–O–Si linkages with terminating Si–OH silanol groups. Increasing the water contact results in the formation of hydrated silica gels and colloidal silica dispersions. Silicic acids exist in the most dilute of aqueous solutions, but these will condense and cross-link to form various polysilicic acids similar to the behavior of borates and phosphates. Hence, although some simple silicic acids have been identified in dilute solutions, such as orthosilicic acid Si(OH)4 and metasilicic acid SiO(OH)2, more complex polysilicic acids are also present.
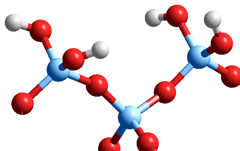
Disilicic acid condensation product of silicic acid
2 Si(OH)4 → Si(OH)3OSi(OH)3 + H2O
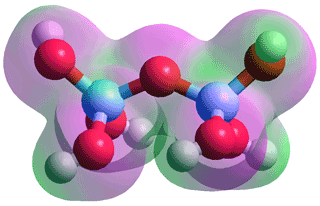
Orthosilicic acid usually called silicic acid, Si(OH)4 see top right) is not found as a solid. It may be formed by aqueous silicon dioxide (silica, SiO2, its anhydride) hydrolysis, but iavoids the reverse condensation only if its concentration is kept very low (~1 mM). e Larger amounts may be made in non-aqueous solutions by the hydrogenolysis reaction of benzyloxysilanes. a Silicic acid has one acid group, pKa = 9.82, d forming silicates, and is more soluble (but more reactive) above this pH (i.e. when more alkaline). It is less soluble (slowly condensing and dehydrating to form SiO2) below the pKa except at very acid pH's (pH ~ 0). The Si(OH)4 polymerization is shown right [4027].
Alpha-quartz
The reaction proceeds as a transfer of a proton between the Si(OH)4 molecules, followed by the nucleophilic attack of the negatively charged O atom on the positively charged Si atom resulting in the elimination of an H2O molecule. Due to the several (but equivalent) silanol groups present, the polymerization can produce chains, rings, two-dimensional and three-dimensional networks, and eventually forming gels. b Condensation of the silicic acid molecules tends to maximize the number of Si-O-Si bonds by preferably forming cyclic species. Also, a process of Ostwald ripening occurs whereby smaller particles redissolve to redeposit on larger particles. Dissolved cations affect this process and the resultant structures of the oligomers and polymers. The pKa of the oligomers differs little from that of Si(OH)4 but the pKas become lower on the surface of nanoparticles. c
Silica (SiO2) with some surface silanol groups,
the tetravalent Si atoms are light blue
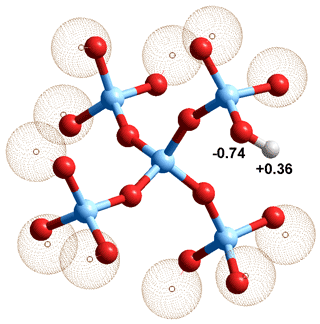
Silica dissolves extremely slightly in water with contacting aqueous solutions containing up to mM dissolved silica (<120 mg ˣ L−1 at 25 °C). This slight dissolution has to be allowed for when undertaking precision work such as the determination of water's triple point. This dissolution becomes significantly greater at higher temperatures where Si(-O-H)4 is formed [3856] and may enter the gas phase. 'Soluble' or 'dissolved' silica contains reacting mixtures of monomers, dimers, and polymers of silicic acid whereas 'insoluble' or 'colloidal' silica results from silicic acid polymerization. In an aqueous solution the Si(O-H)4 is directly hydrated by eight H2O molecules, some of which may form four-membered Si-rings, including two hydrogen bonds with the geminal silanol groups (see below left).
Si(-O-H)4.(H2O)4
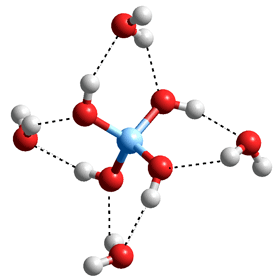
The O-Si-O (O···O, ~2.6 Å) angle is rigidly but approximately tetrahedral (~109°). However, the Si-O-Si angle is highly flexible (Si···Si, ~3.2 Å), and in contrast to organic ethers can become linear with little energetic cost, but centered on 142°. Although some silicas are crystalline, most are amorphous and glassy materials.
Surface silanol (ºSi-O-H) groups
Silicas interact, with water and biomolecules, at their surfaces mainly using their silanol groups (Si-O-H) [1109, 3855]. These are formed on the exposure of a new silica surface to water vapor. The surface ºSi-O-H groups (see left) may ionize;
ºSi-O-H + H2O ![]() ºSi-O− + H3O+
ºSi-O− + H3O+
with pKa1 ≈ 5.7 (~40%; 25 °C; low ionic strength) or pKa2 ≈ 8.5 (~60%; 25 °C; low ionic strength) [3854]. These
pKa's are temperature dependent, dropping with an increase in temperature and with both pKa's equal at ~ 50 °C (pKa1 = pKa2 = 4.3). Any produced surface silanone groups ( >Si=O) rapidly convert to the geminal silanol ( >Si(-O-H)2 ) at aqueous surfaces. The triple silanol (-Si(-O-H)3 ) is unknown on these surfaces.
Si(-O-H)4.(H2O)12, where each of the six pairs of
geminal silanol groups are joined by a hydrogen-
bonded water dimer
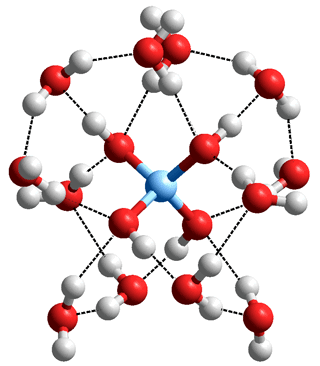
All surface silanol groups are hydrophilic and will hydrogen bond to H2O. Some of these hydrating water molecules may span pairs of close silanol groups (e.g., vicinal, H-O-Si-O-Si-O-H) with an H2O molecule as both proton donor and acceptor to geminal silanol ( >Si(-O-H)2 ) groups.
Fully hydroxylated surfaces contain about 4 - 5 Si−O-H nm−2. The surface Si-O-Si ethers are far less hydrophilic
and act like hydrophobic areas. Crystalline silica, therefore, possesses some hydrophilic-face surfaces and some hydrophobic-face surfaces. Dipolar interactions from such hydrophobic faces cause increasing attraction towards the interface resulting from the changed molecular orientations of the direct solvating water molecules. This surprisingly exerts a longer-ranged attraction of water molecules towards the hydrophobic quartz interface than to the hydrophilic interface [4041]. The dipoles of the bound water molecules will, on average, point away from a neutral silica surface due to their hydrogen bonding orientations. The ionization of the silanol groups reverses this arrangement. Where water is present, tightly bound water layers cover all silica surfaces. Angle-resolved vibrational sum-frequency generation experiments have shown that the silica-water interface contains highly ordered water molecules at low ionic strengths [3862]. Using surface-selective vibrational spectroscopy, it has been proposed that water molecules gather around the silanol groups to form strongly hydrogen-bonded droplets. At high humidity, deprotonation of the silanol was not observed, unlike the case of silica surfaces in
contact with bulk liquid water [4169].
At the charged silica surface, the close average spacing between oxygens in the glass surface (~2.6 Å) (cf., that in water, ~2.8 Å) creates a significantly different environment for H-bond lifetimes, shorter hydrogen bonds, faster proton transfers, and newly formed H3O+ ions adjacent to the interface [3737]. The presence of ions near the silica-aqueous interface screens the surface charge and increases the surface hydrophobicity by shifting its structural makeup from dominant water-surface interactions to water-water interactions [3972].
The incorporation of water and trace elements into silica matrices has been studied [4038]. Water defects were associated with trace elements and may be trapped during the formation of quartz and may disintegrate into water-containing bubbles saturated with basic radicals and acidic and volatile elements. Such regularly packed fluid inclusions are observed in opal. Whereas the presence of cations (particularly Fe3+) or faults derived by irradiation, give the various color modifications of quartz, such as citrine, rose quartz, amethyst, and smoky quartz.
The natural oxidation, of silicon (Si0) surfaces, depends on their aging conditions, gradually becoming hydrophilic (through >Si=O) and then attaching a strongly hydrogen-bonded water network (through >Si(OH)2) in ambient air. However, under alcohol, the surface remains hydrophobic and unoxidized [3932].
Silica surfaces can be made hydrophobic by methylation (or similar), replacing all or most of the surface hydroxyl groups with methylsilyl ones. Thus, such silicas are not wetted with water. However, mixtures of these hydrophobic materials with (underivatized) hydrophilic silicas and water give rise to homogeneous mixtures where the air is replaced by water within the hydrophobic particles [4198].
[Back to Top ![]() ]
]
a M. Crashing, T. Matsumoto, F. Hashish, H. Latashia, T.Ohhara, T. Hanashima, A. Nakao, T. Moyoshi, K. Sato1 and S. Shimada, Non-aqueous selective synthesis of orthosilicic acid and its oligomers, Nature communications, 8 (2017) 140, DOI: 10.1038/s41467-017-00168-5. [Back]
b P. Innocenzi, From silicate oligomers to gelation, In, The Sol-to-Gel Transition, SpringerBriefs in Materials, Springer Nature Switzerland (2019) DOI: 10.1007/978-3-030-20030-5_5; L. Lunevich, Aqueous silica and silica polymerisation, IntechOpen, (2019) doi :10.5772/intechopen.84824. [Back]
c D. J. Belton, O. Deschaume and C. C. Perry, An overview of the fundamentals of the chemistry of silica with relevance to biosilicification and technological advances, FEBS Journal. 279 (2012) 1710-1720. [Back]
d Three fother pKa s have been reported (11.8,12, 12), but this 1954 data has not been reproduced recently. D. D. Perrin, Dissociation constants of inorganic acids and bases in aqueous solution, http://publications.iupac.org/pac/pdf/1969/pdf/2002x0133.pdf. [Back]
e On concentration above ~2 mM, silicic acid, Si(OH)4 solutions form silica gel; an initially wet and extensive gel but finally an amorphous and porous form of silica, with small (~2 nm) voids and pores. [Back]
Home | Site Index | Aqueous H2S | Aqueous hydrogen halides | Aqueous ammonia | Aqueous borate | LSBU | Top
This page was established in 2020 and last updated by Martin Chaplin on 25 June, 2021