
Corn, a good source of starch

Starch is an inexpensive thickener, water binder, and gelling agent.
Starch is the most abundant biomolecule on earth after cellulose and the major carbohydrate reserve in plant tubers and seed endosperm. It is found as granules [330, 1758, 3612] each typically containing several million amylopectin molecules accompanied by a much larger number of smaller amylose molecules. By far, the largest source of starch is corn (maize), with other commonly used sources being wheat, potato, tapioca, and rice. Amylopectin (without amylose) can be isolated from 'waxy' maize starch, whereas amylose (without amylopectin) is best isolated after specifically hydrolyzing the amylopectin with pullulanase [405]. Genetic modification of starch crops has led to the development of starches with improved and targeted functionality [593].
Starch consists of two molecular families, amylose (typically 20-30%) and amylopectin (typically 70-80%). Both consist
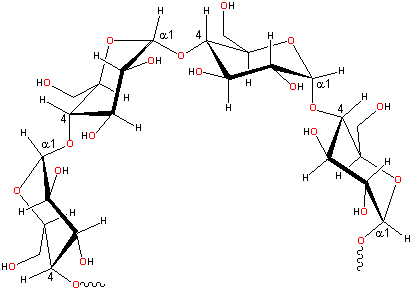
of polymers of α-D-glucose units in the 4C1 conformation. In amylose, these are linked a-(1![]() 4)-,
with the ring oxygen atoms all on the same side, whereas in amylopectin,
about one residue in every twenty or so is also linked a-(1
4)-,
with the ring oxygen atoms all on the same side, whereas in amylopectin,
about one residue in every twenty or so is also linked a-(1![]() 6)-
forming branch-points. The relative proportions of amylose to amylopectin
and a-(1
6)-
forming branch-points. The relative proportions of amylose to amylopectin
and a-(1![]() 6)- branch-points both depend
on the source of the starch; for example, amylo-maizes contain over
50% amylose, whereas 'waxy' maize has almost none (≈ 3%) [260].
6)- branch-points both depend
on the source of the starch; for example, amylo-maizes contain over
50% amylose, whereas 'waxy' maize has almost none (≈ 3%) [260].
Representative partial structure of amylose
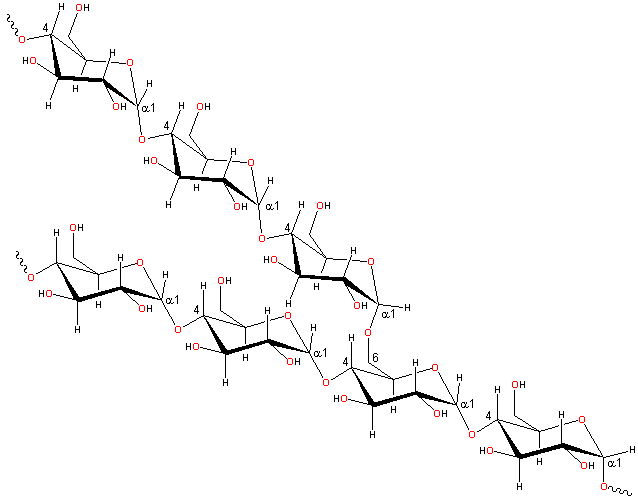
Representative partial structure of amylopectin
[Back to Top ![]() ]
]
Chain lengths after debranching starch, from [4157].,
both the amylopectin and amylose chains are bimodal.
![Chain lengths after debranching starch, from [4157], both the amylopectin and amylose chains are bimodal. Chain lengths after debranching starch, from [4157], both the amylopectin and amylose chains are bimodal.](images/amyl.gif)
Amylose and amylopectin are inherently incompatible
molecules; amylose has lower relative molecular mass (molecular weight ~ 106 Da) with a relatively
extended shape, whereas amylopectin has huge but compact molecules (molecular weight ~ 107 - 108 Da).
Both amylose (in particular) and amylopectin may shear during preparation. Determination of the molecular mass distribution of starch molecules presents several problems [1593]. The presence of amylose reduces the crystallinity of the amylopectin and influences the ease of water penetration into the granules. Most of their structure consists of a-(1![]() 4)-D-glucose
units. Although the α-(1
4)-D-glucose
units. Although the α-(1![]() 4)
links are capable of relatively free rotation around the (φ) phi and (ψ) psi torsions, hydrogen bonding between
the O3', and O2 oxygen atoms of sequential residues tends to encourage
a helical conformation. These helical structures are relatively
stiff and may present contiguous hydrophobic surfaces.
4)
links are capable of relatively free rotation around the (φ) phi and (ψ) psi torsions, hydrogen bonding between
the O3', and O2 oxygen atoms of sequential residues tends to encourage
a helical conformation. These helical structures are relatively
stiff and may present contiguous hydrophobic surfaces.
Amylose molecules consist of single mostly-unbranched chains
with 500-20,000 α-(1![]() 4)-D-glucose
units dependent on the source (a very few α-1
4)-D-glucose
units dependent on the source (a very few α-1![]() 6
branches and linked phosphate groups may be found [258],
but these have little influence on the molecule's behavior [330]).
Amylose can form an extended shape (hydrodynamic radius 7-22
nm [263]) but generally
tends to wind up into a rather stiff left-handed single helix
or form even stiffer parallel left-handed double helical junction
zones (Jmol, 39 KB, [339]).
Single helical amylose has hydrogen-bonding O2 and O6 atoms
on the outside surface of the helix with only the ring oxygen pointing
inwards. Hydrogen-bonding between aligned chains causes retrogradation
[3269] and releases some of the bound water (syneresis). The aligned
chains may then form double-stranded crystallites that are resistant
to amylases. These possess extensive inter-strand and intra-strand
hydrogen bonding, resulting in a relatively hydrophobic structure
of low solubility. The amylose content of starches is thus the
primary cause of resistant starch formation (RS3, see below).
6
branches and linked phosphate groups may be found [258],
but these have little influence on the molecule's behavior [330]).
Amylose can form an extended shape (hydrodynamic radius 7-22
nm [263]) but generally
tends to wind up into a rather stiff left-handed single helix
or form even stiffer parallel left-handed double helical junction
zones (Jmol, 39 KB, [339]).
Single helical amylose has hydrogen-bonding O2 and O6 atoms
on the outside surface of the helix with only the ring oxygen pointing
inwards. Hydrogen-bonding between aligned chains causes retrogradation
[3269] and releases some of the bound water (syneresis). The aligned
chains may then form double-stranded crystallites that are resistant
to amylases. These possess extensive inter-strand and intra-strand
hydrogen bonding, resulting in a relatively hydrophobic structure
of low solubility. The amylose content of starches is thus the
primary cause of resistant starch formation (RS3, see below).
Single helix amylose (VH-amylose) behaves similarly to the cyclodextrins by possessing a hydrophilic exterior surface with a distinctly hydrophobic inner channel (0.54 nm) of similar width to α-cyclodextrin (0.56 nm). This inner channel holds a spiral of water molecules, which are relatively easily lost to be replaced by hydrophobic lipid or aroma molecules when it forms a V-type structure that varies in diameter and packing depending on the size of intrahelical included molecules [2141]. It is also responsible for the characteristic binding of amylose to chains of charged iodine molecules. For example, the polyiodides have chains of I3− (see [4468]) and I5− forming structures such as I93− and I153−. Each turn of the amylose helix holds about two iodine atoms with the blue color being produced by the donor-acceptor interaction between water and the electron deficient polyiodides. Thus, the hydrophobic channel of VH-amylose serves as an excellent steric and hydrophobic fit for the linear polyiodide chain [2950]. Note that neutral I2 molecules may give polyiodides in an aqueous solution, but there is no interaction with neutral I2 molecules except under strictly anhydrous conditions.
[Back to Top ![]() ]
]
Amylopectin model structure
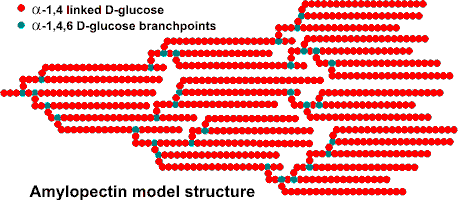
Amylopectin is formed by non-random α-1![]() 6
branching of the amylose-type α-(1
6
branching of the amylose-type α-(1![]() 4)-D-glucose
structure. This branching is determined by branching enzymes
that leave each chain with up to 30 glucose residues. Each
amylopectin molecule contains a million or so residues, about
5% of which form the branch points. There are usually slightly
more 'outer' unbranched chains (called A-chains) than 'inner'
branched chains (called B-chains). There is only one chain
(called the C-chain) containing the single reducing group.
4)-D-glucose
structure. This branching is determined by branching enzymes
that leave each chain with up to 30 glucose residues. Each
amylopectin molecule contains a million or so residues, about
5% of which form the branch points. There are usually slightly
more 'outer' unbranched chains (called A-chains) than 'inner'
branched chains (called B-chains). There is only one chain
(called the C-chain) containing the single reducing group.
A-chains generally consist of between 13-23 residues [1479]. There are two main fractions of long and short internal B-chains with the longer chains (greater than about 23-35 residues) connecting between clusters and the shorter chains similar in length to the terminal A-chains [1479].
Each amylopectin molecule contains up to two million glucose residues in a compact structure with a hydrodynamic radius of 21-75 nm [263] (waxy maize amylopectin >300 nm [1683]). The molecules are oriented radially in the starch granule. As the radius increases, so does the number of branches required to fill up the space, forming concentric regions of alternating amorphous and crystalline structure. In the diagram below: A - shows the essential features of amylopectin. B - shows the organization of the amorphous and crystalline regions (or domains) of the structure generating the concentric layers that contribute to the “growth rings“ that are visible by light microscopy. C - shows the orientation of the amylopectin molecules in a cross-section of an idealized entire granule. D - shows the likely double helix structure [2951] taken up by neighboring chains, giving rise to the extensive degree of crystallinity in the granule. There is some debate over the form of the crystalline structure, but it appears most likely that it consists of parallel left-handed helices with six residues per turn. An alternative arrangement of interconnecting clusters has been described for some amylopectins [1193].
Structure of starch granules
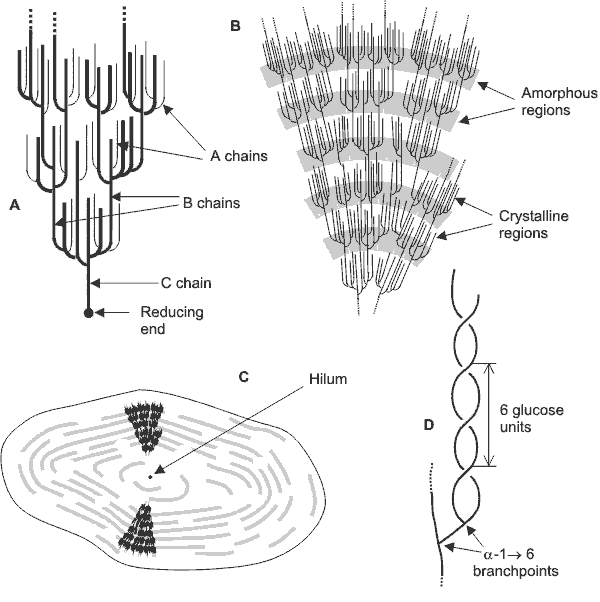
Type A and B amylopectin crystallites
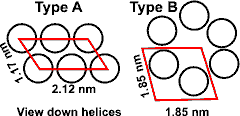
Some amylopectin (for example, from potato) has phosphate groups attached to some hydroxyl groups, which increase its hydrophilicity and swelling power.a Amylopectin's double-helical chains can either form the more open hydrated Type B hexagonal crystallites or the denser Type A crystallites, c with staggered monoclinic packing, dependent on the plant source of the granules [263]. Type A, with unbroken chain lengths of about 23-29 glucose units, is found in most cereals.
Starch is a versatile and cheap material. It has many uses as a thickener, water binder, emulsion stabilizer, and gelling agent. Its form and functionality have been reviewed [1556]. Starch is often used as an inherent natural ingredient, but it is also added for its functionality. It is naturally found tightly and radially packed into dehydrated granules (about one water per glucose) with origin-specific shape and size (maize, 2-30 μm; wheat, 1-45 µm; potato, 5-100 μm [593]). The size distribution determines its swelling functionality, with granules being generally either larger and lenticular (lens-like, A-starch) or smaller and spherical (B-starch) [1118] with less swelling power a. Granules contain 'blocklets' of amylopectin containing both crystalline (~30%) and amorphous areas. As they absorb water, they swell, lose crystallinity, and leach amylose. The higher the amylose content, the lower is the swelling power, and the smaller is the gel strength for the same starch concentration. To a certain extent, however, a smaller swelling power due to high amylose content can be counteracted by a larger granule size [260]. Although the properties of starch are naturally inconsistent, being dependent on the vagaries of agriculture, there are several suppliers of consistently uniform starches as functional ingredients. There is a review of starch modifying enzymes [4088].
Of the two components of starch, amylose has the most valuable functions as a hydrocolloid. Its extended conformation causes the high viscosity of water-soluble starch and varies relatively little with temperature. The extended loosely helical chains possess a relatively hydrophobic inner surface that is not able to hold water well, and more hydrophobic molecules such as lipids and aroma compounds can easily replace this. Amylose forms useful gels and films. Its association and crystallization (retrogradation) on cooling and storage decreases storage stability causing shrinkage and the release of water (syneresis). Increasing amylose concentration decreases gel stickiness but increases gel firmness. Retrogradation is affected by lipid content, amylose/amylopectin ratio, amylose chain length, and amylopectin, and solid concentration [1574]. Amylopectin interferes with the interaction between amylose chains (and retrogradation), and its solution can lead to an initial loss in viscosity and followed by a more slimy consistency. Amylopectins can also partially crystallize, forming gels through double-helical structures with external chains of adjacent molecules. Both external and internal chains form helical inclusion complexes giving rise to effects on the functional properties of the starch, with apparently slight differences in the length of segments having substantial effects on gelling [2582].
Mixing with κ-carrageenan, alginate, xanthan gum, and low molecular mass sugars can also reduce retrogradation. At high concentrations, starch gels are both pseudoplastic and thixotropic with greater storage stability. Their water-binding ability (high but relatively weak) can provide body and texture to foodstuffs and encourages its use as a fat replacement.
Some starch in the average
diet is slowly digestible (SDS) with a significant proportion, labeled 'resistant starch' (RS), escaping degradation in the stomach and small intestine (for reviews, see [991]). This portion is difficult to measure (see [1661] for methods) and depends on a number
of factors including the form of starch and the method of cooking
prior to consumption. Nevertheless, resistant starch serves as
a primary substrate source for colonic microflora and may
have several important physiological roles (see hydrocolloids
and health). Resistant starch has been categorized as physically
inaccessible (RS1), (raw) ungelatinized starch (for example, in banana; RS2 b ), thermally stable retrograded starch
(for example, as found in bread, especially stale bread, mainly
amylose; RS3) and chemically modified starch (RS4).
Resistant starch should be considered a dietary fiber. Although
not exactly quantifiable due to its heterogeneous nature, some
are determined by the official Association of Official Agricultural
Chemists (AOAC) method. Starch with structure intermediate between the more crystalline resistant starch (for example, RS3 in staled bread) and more amorphous rapidly digestible starch (for example, in boiled potato) is slowly digestible starch [293] (for example, in boiled millet). Slowly digestible starch gives reduced postprandial blood glucose peaks and is therefore valuable for the diabetic diet. As consumption of resistant starch and slowly digestible starch is recommended to prevent diseases related to malnutrition, such as obesity, overweight, diabetes, and cardiovascular illness, ways for their increased production are in development [3117].
Many functional derivatives of starch [3552] are marketed, including cross-linked, oxidized, acetylated, hydroxypropylated, and partially hydrolyzed material. For example, partially hydrolyzed (that is, about two bonds hydrolyzed out of eleven) starch (dextrin [1750]) is used in sauces to control viscosity.
Interactive structures are available (Jmol). [Back to Top ![]() ]
]
a Swelling power is determined after heating the starch in excess water and is the ratio of the wet weight of the (sedimented) gel formed to its dry weight. It will depend on the processing conditions (temperature, time, stirring, centrifugation) and may be thought of as its water-binding capacity. [Back]
b The amount of resistant starch is highest in unripe green bananas (≈ 15%) and drops during the ripening to much lower values as the starch is converted to glucose. [Back]
c A-type has monoclinic unit cell parameters of a = 21.24 Å, b = 11.72 Å, c = 10.69 Å, and α = β = 90°, γ = 123.5°. It contains two double helices and two water molecules per glucosyl unit. The packing of adjacent double helices has a translation by c/2 in the unit cell. B-type has a hexagonal unit structure with approximate unit cell parameters, a = b = 18.5 Å, c = 10.4 Å, and a = ß = 90°, ? = 120°, containing two double helices and six water molecules per glucosyl unit. Adjacent double helices are packed without translation with respect to the c-axis. Both types are reported as having gauche-gauche hydroxymethyl groups but, based on vibrational sum frequency generation (SFG) spectroscopy, they may be different [2141]. [Back]
Home | Site Index | Hydrocolloids | Polysaccharide hydration | hydrogen-bonding | LSBU | Top
This page was established in 2001 and last updated by Martin Chaplin on 15 July, 2022