
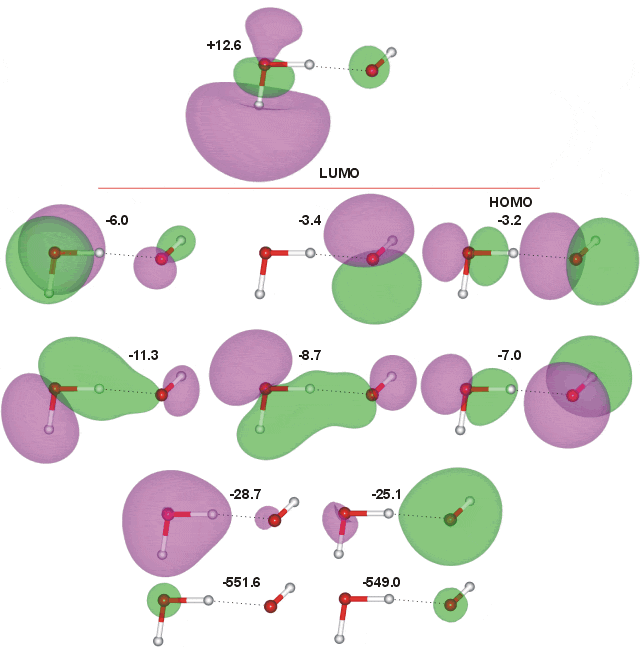
The molecular orbitals were calculated using the Restricted Hartree-Fock wave function (RHF) using the 6-31G** basis set. The calculated energies are in eV. There is more molecular orbital overlap between the molecules than in the water dimer, indicating the stronger hydrogen-bonding.
Interactive structures with orbitals are available (COW [Plug-in, ActiveX] only)
Home | Site Index | Water dissociation | H3O+ and OH− molecular orbitals | H5O2+ molecular orbitals | Hydrogen ions | Hydroxide ions | LSBU | Top
This page was established in 2001 and last updated by Martin Chaplin on 5 August, 2021