
One leaf of a hydrated membrane bilayer made of
1,2-dipaltmitoyl-sn-glycero-3-phosphatidylcholine
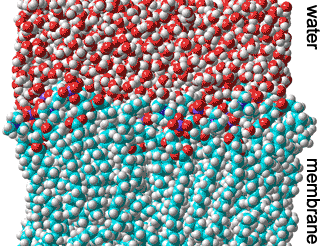
Biological membranes primarily consist of proteins, lipids, and water.
![]() Cholesterol and membrane hydration
Cholesterol and membrane hydration
Life requires water
Lipid membranes require water to form and act
Biological membranes are universal structural units in living cells and organelles, They form a structurally integral part of the whole-cell complex [4166] with natural hydrophobic barriers responsible for the selective transport of materials, the generation of energy, recognition, and signaling. They consist of phospholipid bilayers that contain proteins and other molecules. The phospholipid hydrocarbon chains dominate the bilayer’s flexibility, while the lipid head-groups govern bilayer packing and the interactions with water and other molecules and ions.
The insolubility of phospholipids in water drives lipid self-assembly into membranes, allowing minimum exposure of the hydrophobic parts with water while solvating the hydrophilic phosphate 'head' groups. The formation of 'flat' membranes, as opposed to tightly curved micelles, comes about due to the rectangular lipid structure and the inclusion of proteins. The strongly-held hydrophilic coating forms a barrier to the facile fusing of membranes. Some phospholipids are preferably found on particular sides of the membrane; for example, phosphatidylserine is always found on the inside of plasma membranes [3006]. This is due to the hydration compatibility of the local head groups. Perhaps surprisingly, the most prevalent molecules in the structuring of biomembranes are water molecules [3576]. This may be analyzed using terahertz (THz) spectroscopy [4364].
Early descriptions of membranes revolved around the 'fluid mosaic model' [2592a] (see figure below), where the 'backbone' of the membrane is the phospholipid bilayer. Over the years, further studies demonstrated the need for a more complex model description (as in [2592b]). However, this newer model is still misleading as it does not include the bound and included water, which is essential for biomembranes' formation, structuring, dynamics, stability, and varied biological functions. It is now recognized that water forms an essential, integral, and major constituent in the highly complex structure of biologically active membranes [2767]. This water has very different properties than bulk water.
Contemporary picture of the Fluid Mosaic Model, from
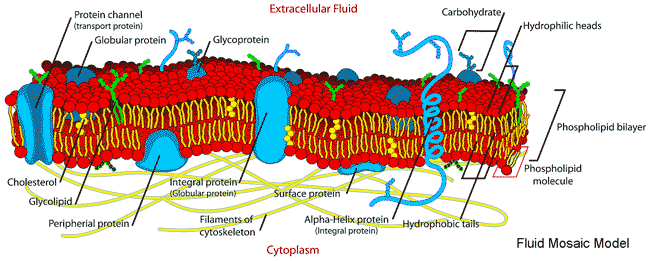
Membranes divide aqueous environments and, amongst other activities, control the passage of materials between them, with water permeation critical to osmotic equilibrium. They are built from a bilayer of phospholipids consisting of a hydrophilic head group within the aqueous environment on both sides and hydrophobic fatty acid 'tails' within the membrane interior. In the model above, sections through the membrane are shown. These hydrophobic areas are not allowed to contact water, so such membranes have no edges, forming continuous surfaces with an outside and an inside. The phospholipids may diffuse laterally, giving rise to the 'fluidity' of the membrane (with the membrane behaving like a 2-dimensional liquid) but only extremely rarely change sides of the membrane ('flip-flop'). Although often ignored, the membrane lipids and proteins' biological functions, structuring, and movement depend substantially on their hydration [2597] and the contacting aqueous phases. For example, water molecules hydrogen-bond between the carbonyls of neighboring dipalmitoyl phosphatidylcholine groups to spread out the head groups and enable them the room to twist such that their dipole moments are in a nearly anti-parallel arrangement [2597]. Membrane proteins often have strongly hydrogen-bound helices for their primary structural support, with the helices usually either lying on the surface and parallel to the membrane surface or perpendicular to the surface and crossing the membrane. Due to their hydrophobic environment, these backbone hydrogen-bond strengths may be as much as 25 kJ ˣ mol−1 greater than the water-water hydrogen bonds [3044]. Where they are weaker, they indicate a bending or cleavage site in the protein's function or activity [3044]. The hydration layer controls lipids' mobility in membranes, which may be as small as six to seven molecules per lipid head group 4335].
Cell membranes are heterogeneous, being self-assembled from hundreds of different lipids plus many different membrane proteins and other materials, such as cholesterol. The lipids are able to move, with their associated water, within their two-dimensional surface. Their surfaces are highly corrugated (not planar as often displayed in the fluid mosaic model, see above, which would result in slow perpendicular motion relative to lateral diffusion), resulting in the very reduced lateral diffusion of the water and other molecules along the surfaces [2589], as such lateral motion also involves perpendicular motion pushing other molecules out of the way. The ratio of the lateral to the perpendicular motion will depend on the membrane topographical and chemical structure at issue. Adequate hydration is needed for the structure, dynamics, and function of the membrane proteins, not only at their external aqueous interfaces but also within the membrane [2591], and with hydration also needed for the fluidity (as a plasticizer) of the phospholipid biomembrane itself. Hydration also mediates adhesion and fusion between different membranes and between membranes and proteins, plus its importance in membrane protein structure and activity. The aqueous interface next to the membranes forms a functional unit linking the membrane to the water-soluble proteins of the cytosol, facilitating the rapid exchange of structural, dynamic, and physiological information [1553]. Collective and correlated vibration motions, mediated by the intervening hydrating water molecules, persist up to distances of 2.5 nm between the membrane and protein surfaces [3593].
However, the complexity of membrane structure does not mean that they are randomly organized as different areas of a membrane will have different constituents, and the different phospholipids are tightly controlled (although this is currently poorly understood) [2765]. This diversity makes the study of membrane hydration difficult with most 'model,' studies involving membranes constructed from a single phospholipid type. The results of such studies (some given below) can only be indicative of the biological reality. Also, it should be remembered that gases such as oxygen, hydrogen, and nitrogen are much more soluble in the oily part of biomembranes than in water and may have an (unexpected) effect on their properties.
1-Palmityl-2- oleyl phosphatidylethanolamine
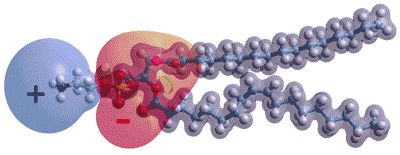
Opposite shows the charge distribution in a typical phospholipid molecule, palmitoyl oleoyl phosphatidyl-ethanolamine. a The ionic groups generate strong electric fields in the plane of the membrane and will interact with neighboring head groups and imposed electric fields. In the absence of proteins, most lipid membranes are expected to be negatively charged (i.e., negative zeta potential) due to the steric arrangement with more closely held hydrogen phosphate anions, together with counter-ion attraction that allows anions closer than cations. However, they will allow proton ransfer in the plane of the surface by hopping between anion groups. They have the capability of being directed by differences in the head groups within different raft (dynamic domains > 40 nm in size, [4025]) areas. The proton conductivity along the membrane is vital for cellular bioenergetics. These surface protons are not rapidly equilibrated away towards the bulk.
Asymmetry in the number of lipids per side (inner and outer leaflet) of a membrane may be caused by hydrogen-bond interactions and hydration between the head groups [3006]. Thus, they affect these raft areas [2634]. The different sides of biomembranes are dissimilar with different phospholipid and protein compositions [3006].
Examples of phospholipids,
R1 and R2 are fatty acid chains
As most biomembranes present curved surfaces, they are naturally asymmetric with the inner leaflet being more tightly curved, than the outer leaflet, and with a lesser surface area. This asymmetry may be achieved by a different number of lipids in the outer and inner leaflet, a difference in transmembrane head-group hydration or a different head group orientation for the interacting phosphate groups [2962]. Both sides involve highly hydrophilic head groups of phospholipids, with some examples given left. The hydrophobic tail groups (R1 and R2 left) are also varied and often consist of one saturated and one kinked unsaturated group in each phospholipid (see palmitoyl oleoyl phosphatidyl ethanolamine above). Due to their different positioning in the glycerol backbone, the hydrophobic chains are axially displaced by about two methylene (CH2) units towards the middle of the bilayer. It follows that molecules that interfere with surface hydration also change the local membrane curvature, possibly further affecting the biomembrane enzymology and transport properties [3006].
The head-groups extend to different extents away from the bilayer according to their structures, charges, and neighboring steric and electrostatic interactions. They strongly bind many water molecules in subtly different manners, and their interfaces are somewhat rugged and heterogeneous with highly variable diffusibility, with some water being trapped and almost static [2585]. The number of water molecules associated with each head group varies with the head group, its neighbors, and the method used in the determination. In particular, the negatively charged phospholipids such as phosphatidyl-serine and phosphatidyl-inositol, although generally in the minority, appear to have very important roles in stiffening the membranes and attracting and binding the proteins to the membrane surface; processes modulated by cations [2754].
The density of material through a DPPC membrane.; from [2586]
![Density of material through a 1,2-dipaltmityl-sn-glycero-3-phosphocholine membrane.; from [2586] Density of material through a 1,2-dipaltmityl-sn-glycero-3-phosphocholine membrane.; from [2586]](images/membrane.gif)
Shown on the right is the density profile of material across a 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine membrane [2586] (DPPC), used as an example. d The molecular structure beneath the graphs is indicative only, with the actual 3-dimensional structure folding up in different ways, causing about a 10% shortening in length and giving rise to corrugated (i.e., not planar) surfaces. It shows how the water density (blue) mixes with the phosphate (violet) and carboxylate (red) in the head-groups but reduces up to the methylene chain of the fatty acid (gray) with effectively none within the interior. Both the water diffusion rate [2789] and the water activity reduces (qualitatively rather than quantitatively) in line with the water density line (blue). The reduction in the water motions together with the decrease in the entropy of the water near the phospholipid bilayers, is extended by the presence of a peripheral membrane-bound protein. These effects range up to about a nanometer above that protein aiding molecular recognition, binding, and protein−protein interactions [2789]. Similar to that occurring in proteins, a dynamical transition in the bilayer interior is found at about 188 K using 2H electron spin echo envelope modulation (ESEEM) NMR-like spectra. This is due to the onset of isotropic water movement. Another transition at about 100 K is ascribed to water's restricted reorientational motions and lipid chain flexibility around defective free-volume holes [2923].
The water activity also varies parallel to the membrane, across its surface, due to differences between the phospholipids and the presence of membrane proteins. This causes variations in the lipid packing and the flow of water.
The head-groups are marked by higher absolute density due to greater oxygen and phosphorous density plus more densely held water. The middle of the membrane shows slightly lower absolute density due to the chain ends with associated vacant space caused by their poor interactions. The surface area taken up is about ½ nm2 which increases by about 1% °C−1. About 10-12 H2O molecules may be included within the head group volume, with half of these associated with the phosphate and any remaining affected water molecules lying above this surface. Using molecular dynamics, these affected water molecules are shown to stretch at least two further molecular diameters (≈ 10-12 H2O) before there is a little discernible effect on their diffusivity [2585]. Using terahertz spectroscopy, three types of water have been found amongst the lipid head groups; (1) bulk-like, (2) frozen held firm by two or more hydrogen bonds, and (3) molecules with only one (or perhaps no) hydrogen bond allowing rapid rotational diffusion in more hydrophobic areas [2587]. The surface area of the head groups depends on their charge interactions with neighboring similarly charged groups expanding this area (like charges repelling) with the influx of water molecules. The effect of mixed charged headgroups depends on their neighbors (unlike charges attract) [3519].
Molecular dynamics simulations have shown that continuously residing interfacial water molecules in hydrated dimyristoyl-phosphatidylcholine (DMPC) lipid bilayer form less mobile hydrogen bonds among themselves and concertedly to the oxygens of the carbonyl, phosphate, or the DMPC glycerol head-groups [3244]. These result in the water-mediated
lipid-lipid associations.
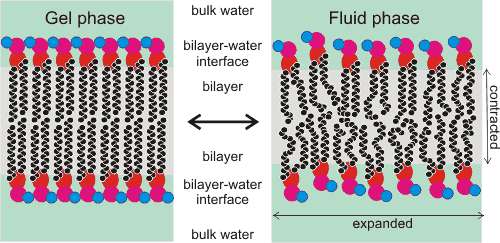
Cartoon showing the gel-fluid transition in phospholipid bilayers
Interactions with water can be estimated from known interactions of other related molecules in the solution. The negatively charged phosphate groups (shown red, right, within the head groups) are expected to hydrogen bond two water molecules strongly through each of their strongly charged singly bonded oxygen atoms and one water molecule less strongly through each of their doubly single-bonded (ether) oxygen atoms (i.e., 6 H2O per lipid). Most of these water molecules are likely to be so strongly bound as to be unfreezable.
The deeply-located carboxylate ester groups within the head groups (shown orange, above right) are expected to hydrogen bond weakly to two water molecules through their doubly bonded (keto) oxygen atoms and one water more weakly through their doubly single-bonded (ether) oxygen atoms.
Melting temperature with water content, from [2605]
![Melting temperature with water content, from [2605] Melting temperature with water content, from [2605]](images/dppcgel.gif)
However, some of these inner hydrogen bonds may be disallowed for steric reasons, particularly as one of the two ester groups is buried deeper into the membrane (i.e., 3- 6 H2O per lipid). These water molecules may (mostly) link to oxygen atoms of neighboring ester or phosphate groups. Water hydrogen-bonded to the phosphate groups also (mostly) hydrogen bonds to other phosphate groups or further water molecules [2766]. Phosphate groups may also form ion-pairs or water-separated ion pairs to cations.
Hydrogen-bonding to the remaining head group depends on its structure, but for the choline group in the example, they are likely to form (freezable) partial clathrate shells (≈ 10-12 H2O per lipid) with no direct hydrogen bonds. Other H2O may be trapped dependent on the neighboring structures. Overall there are about 28 H2O molecules directly affected (using terahertz spectroscopy) per lipid molecule out to at least one nm from the surface (≈ 41 % w/w, xw ≈ 0.97) [2590]. Other molecules further out to about 3 nm will be orientated by the inner water [2963]. The higher osmotic pressure generated by these inner water molecules (>70 MPa, lower water activity <0.5, [1553]) is partially compensated by the surface pressure exerted by and between the head groups, but will extend somewhat into the neighboring aqueous phase to repel approaching similar membranes (as shown by supported lipid membranes). This osmotic force is also known as the hydration force.
If excess water is attracted towards and into the surface, the swelling may result in membrane leakage. If less water is available, the membrane is dehydrated with reduced overall thickness, a reduced area per lipid, reduced permeability, and reduced fluidity [2605], making the membrane sub-optimal or totally inactive. This is particularly noticeable at hydration levels less than 12 H2O per lipid (see figure above left). The membranes represent one of the critical sites of irreversible damage by dehydration, including that caused by ice formation removing the liquid water. However, the lipid structure may be maintained in the dehydrated state if it is dried (or frozen) in the presence of trehalose that binds to the carbonyl groups replacing about half the water molecules per lipid molecule. In this way, trehalose encourages the fluidizing of the dry bilayers by preventing the head groups from directly interacting to form static condensed structures. Although trehalose prevents damage to the membrane, it is insufficient for the active working of the membrane that requires the water molecules' dynamic movement.
Depending on the membrane structure, small amounts of water can enter the membrane due to their small size and their few or zero retaining hydrogen bonds when lying close to the interface. They start by hydrogen-bonding to the ester carbonyl groups, then moving between nano pools created from kinks in the lipid acyl chains. The surface packing may aid this process. This allows the slow movement of water across membranes dependent on the osmotic pressure (water activity) difference between the solutions on opposite sides. This movement may be assisted by the lateral flipping of membrane lipids [3467]. The rate of flow may be different in different directions even if under the same pressure. The disorder entropy can compensate for the water's reduced entropy in the restricted water pools they allow in the acyl chains [2588]. If, however, the membranes are dehydrated, containing less than the required (≈ 20 H2O) water, the bilayer will stiffen, c disallowing such transport. Other molecules can cross the membrane by passive diffusion or via specific transporting membrane proteins, either active or passive [2772]. Passive diffusion depends mostly on the partitioning coefficient of the (hydrophobic) material into the membrane and the concentration difference across the membrane. The acyl tail structures also critically affect the headgroup phosphate orientational distribution and lipid-associated water molecules at the air/water interface, showing the importance of acyl chain chemistry in determining not only membrane fluidity but also membrane hydration [4489].
Aqueous ions affect the surface pressure of DPPC monolayers and are expected to affect biomembranes similarly. The presence of the ions close to the interface was found to increase the surface pressure at a fixed area per molecule, with this increase dependent on the ion in order of the Hofmeister series. Thus SCN− gives a far more significant increase in pressure than Cl−. It is thought that these anions partition into and interact with the head-group volumes but not the hydrophobic tail volumes [2657]. H3O+ ions partition into the water-lipid interface, displacing water and forming strong, long-lived hydrogen bonds with lipid oxygen atoms. This results in a more compact surface area with an increased bilayer thickness [3123].
The curvature of membranes depends on the size and aqueous interactions of the surface polar groups, the volume to length ratio of the hydrophobic tails, transient domains of ordered water induced by divalent ions [3938], and the differences between the two sides of the membranes [3006]. This curvature is an important structural parameter of the membrane that, in turn, plays a key role in the diverse chemistry of the membranes [3666].
There are aqueous nanospaces inside and between cells, surrounded by a cellular membrane, with dimensions of 10-1000 nm, such as mitochondria, prokaryotic cells, and synaptic clefts. Water within such spaces has increased viscosity due to interactions between the water molecules and the lipid bilayer surface [4293].
[Back to Top ![]() ]
]
Like the crowded but aqueous cytosol, most membranes are also crowded with proteins. A high concentration of these are transmembrane proteins such as ion channels. The separation between membrane proteins may be as small as one or two lipid molecules and this slows down the dynamics of the lipid membrane [3935].
Bound water molecules surround biologically essential ions. If water molecules are to pass through biological membranes, then the energy cost of removing these hydrating water molecules while they transit must be compensated. Therefore, the channels must provide environments similar to the aqueous solution. Water wires are critical for the functioning of many membrane proteins, often aided by the protein backbone carbonyl oxygens lining the channels [3966]. Also, some channels must be ion-selective. The channels' pore-lining amino acid residues often arrange a local environment that mimics the local ion hydration environment [3930]. Many of the ion channels are voltage-gated [3931].
[Back to Top ![]() ]
]
Cholesterol
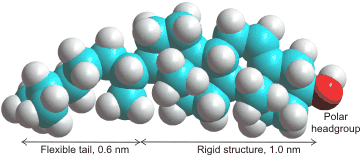
Cholesterolb is found in the membranes of almost all Eukaryotes, where it regulates critical cell functions and is required for life. However, it is absent in Prokaryotes. The amount of cholesterol present depends on the membrane but may vary up to half the molecules present.It is used to make the membrane stronger (condensing its structure), thicker, less fluid, less permeable to hydrophilic and hydrophobic molecules, and particularly to slow down water diffusion through the membrane. Cholesterol seems to have the perfect structure for this purpose both in its small head group and the length of its tail [2610]. Although cholesterol generally stiffens membranes, it has appeared to have far less effect on some structures, such as di-oleoyl-phosphatidyl-choline (DOPC) bilayers [2683]. It does, however, cause local stiffening in DOPC membranes [4068]. Cholesterol also eases transitions in the phase structure of the membranes (packing transitions). This is particularly relevant to the interference of anesthetics with the transmission of the nervous impulses, the changes in phases interfering with the opened and closed ion channels [3006]. Simulated atomistic DPPC:DOPC and DPPC:DOPC:cholesterol lipid bilayers have been used to investigate phase transitions at 310 K to 270 K [3873].
Cholesterol changes the water distribution pattern between tightly and loosely bound water molecules within the membranes [4216].It causes more bulk water to penetrate the lipid interface but with weaker hydrogen-bonding [4480]. It also weakens the interactions between the lipid head-groups and water and thus reduces the number of water- lipid head-group interactions. This leads to a decrease in the velocity (< 100 cm−1) of laterally-propagating vibrations (surface sound waves) [3603]. Thus, the membranes are more capable of dissipating excess energy under mechanical stress.
The transition in phospholipid bilayers on the addition of cholesterol
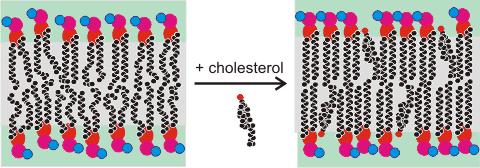
The polar alcohol group sits on the surface at the level of the carboxylic ester groups. It pushes the phospholipid head groups apart so enhancing their (head group) mobility and accelerating the translational diffusion of local water along the membrane surface. Within the bilayer, cholesterol strengthens the hydrophobic hydrocarbon connections so dramatically slowing and reducing penetration by water [2606], hydrophilic or hydrophobic molecules [2607]. Cholesterol may partition into the core of polyunsaturated membranes, where it has faster lateral diffusion [3616].
Contrasting to the effect of cholesterol, water encroachment into lipid bilayers increases slightly (~ 0.1 nm) with the addition of long-chain aliphatic alcohols [3576]. Although small, this increases the water bound by the membrane by about one water molecule per aliphatic alcohol and gives rise to changes in the membrane's properties.
[Back to Top ![]() ]
]
Tripalmitoyl glyceride
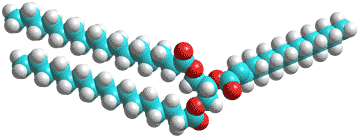
Biological fats and oils consist of mixtures of triacylglycerides (triglycerides, see right); esters derived from glycerol, and three fatty acids. In adipose tissue, they act as a store of energy and (metabolic) water (for example, in camel humps and Namibian bushman's bottoms).
Water has low (< 0.05% v/v) solubility in triglycerides due to their hydrophobicity but can hydrogen bond to the ester oxygen atoms (shown red right) of single triglyceride molecules or hydrogen bond between neighboring molecules [2764]. Thus, they may influence the triglyceride fluidity and packing, as with membranes (above). Also, the water may promote hydrolysis and auto-oxidation reactions. Little has been published of the molecular mechanisms in this area, however. Phospholipids may surround lipid droplets, and these surfaces, in turn, may associate with proteins to regulate lipogenesis and lipolysis to modulate the size of the lipid droplets. The water density in the core of the lipid droplets may be unexpectedly higher than expected (0.8% w/v) [4177].
Water can penetrate triacylglyceride phases and form small pools of water within cavities, with the fat head groups pointing towards each other [3137].
Sebum is found on our skin and regulates our skin hydration and its waterproofing. It includes triglycerides (≈ 45%, e.g., tri-cis-6-hexadecenoin), wax esters (≈ 25%), squalene (a 30-carbon polyene, ≈ 12%), and free fatty acids (≈ 10%, which may increase in response to skin surface bacterial action). The triglycerides form three-dimensional networks of cylinders where the glycerol sub-structures lie within the cylinders together with some water. These act as pathways for water diffusion [3147] to and from the skin.
[Back to Top ![]() ]
]
Micelle particle
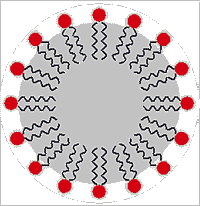
Surfactants (surface active agents) are compounds that preferentially sit at the interface between two liquids (usually water and an immiscible hydrophobic liquid) and lower their surface tension. They may act by themselves or in conjunction with other molecules (co-surfactants, like cholesterol in the case of membranes). They usually have a hydrophilic head-group (shown as a red circle on the right) and a hydrophobic tail (shown as the wiggly lines depicting alkyl groups). In addition to reducing the direct interactions between water and the hydrophobic liquid, surfactants serve to increase the disorder (surface entropy) so reducing the interfacial tension [4423].
AOT
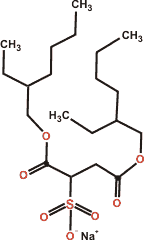
In contrast to many textbook articles, ionic surfactants do not form thin interfacial monolayers at the air-water surface but thicker adsorption layers with the surfactant head-group placed at different depths and associated with their counter-ions [3867].
Regular (water on the outside) or reverse emulsions (water on the inside) with droplet diameters in the range of 10–1,000 nm are called nanoemulsions. They contain micelles (see right) in the same size range as nanobubbles. Nanoemulsions [3128] have a high surface area per unit droplet volume and have growing uses in the food, health, and technology industries. The energy of the micelles depends on their diameter, so the particles' sizes in nanoemulsions are always approximately uniform at equilibrium. As surfactants lie through the surface of emulsions and the emulsion surface area depends on their micelle diameter, this micelle diameter is mostly determined by the relative surfactant to contained liquid concentrations.
The surfactant sodium bis(2-ethylhexyl) sulfosuccinate (AOT, Aerosol-OT) is a versatile surfactant that takes up about 0.75 nm2 area per molecule at the critical micelle concentration [3129b]. As with many similar surfactants, there is little interaction between the hydrophobic parts of neighboring surfactant molecules, with the major interaction being hydrogen-bonding between the surfactant's hydrophilic atoms and water [3129a]. Also, the charged polar sulfonate head-group portion of AOT is highly solvated in an aqueous solution with a net dipole orientation perpendicular to the interfaces at planar interfaces and in normal and reverse nanoemulsions.
Concentrated surfactants form liquid crystals with various structures (e.g., lamellar, hexagonal, cubic) and a wide range of properties and with water activities reducing with increasing relative surfactant concentration [3228].
[Back to Top ![]() ]
]
a Lipid publications rarely use the IUPAC nomenclature where, for instance, palmitic acid is hexadecanoic acid. [Back]
b Cholesterol is a natural steroid that we need to biosynthesize to live. We also get a lesser amount from animal sources in our diet. For many years, however, excessive cholesterol in our diet has been linked to narrowing of the arteries (arteriosclerosis), heart attacks, and strokes, with statins being prescribed to counteract its effects. A (controversial) 2016 study has shown that high levels of low-density lipoprotein cholesterol (LDL-C) in the blood is inversely associated with mortality in most people over 60 years old[2609], a fact that appears inconsistent with the long-standing cholesterol hypothesis. [Back]
c Stiffness (flexibility) of membranes can be described using the bending modulus KC. For an ideal bilayer, the intrinsic curvature is zero, with the energy required to deform the membrane from its intrinsic curvature to some other curvature Ebend (r) given by
Ebend (r) = KCCx,y
2/2 = (KC/2)![]()
where Cx,y is twice the mean curvature at location (x,y) in the plane of the membrane that has fluctuations in the z-direction (see [2683] for more details).[Back]
d The fully hydrated 1,2-dimyristoyl-sn-glycero-3-phosphorylcholine (DMPC) lipid bilayer has also been simulated atomistically in the presence of the TIP4P/2005 water model at 308 K [4172]. [Back]
Home | Home | Site Index | Polysaccharide hydration | Protein hydration | Nucleic acid hydration | LSBU | Top
This page was established in 2016 and last updated by Martin Chaplin on 27 August, 2022