
'a new truth passes through three stages: it first says that it is not true, then that it is contrary to religion,
and finally, that we knew it before'
attributed to Louis Agassiz before 1863,
'Because of the importance of water in the theoretical speculations as well as the practical routine of chemists,
a review of the theories concerning its structure is pertinent.'
Harris Marshal Chadwell 1927
![]() Is liquid water one liquid or two?
Is liquid water one liquid or two?
![]() Are the icosahedral clustering model and the outer structure two-state
mixture models related?
Are the icosahedral clustering model and the outer structure two-state
mixture models related?
The anomalous properties of water have excited chemists and physicists in their attempts to find explanations for many years. Frank concluded in 1970 that physicists were mostly drawn to 'uniformist' models in which all molecules must be regarded as equivalent, whereas chemists generally preferred 'mixture' models [767j]. This conflict has continued to the present day, with entrenched positions on both sides. However, an initially slow conversion towards the acceptance of the mixture models is becoming increasingly noticeable of late. The mixture hypotheses started out with ice-like permanent features, but this has changed over the years into rapidly 'flickering' clusters with no permanent multi-molecular forms but with long-lasting clustering arrangements where molecules rapidly come and go. Hypotheses that have put forward have mainly involved consideration of different forms of amorphous ice [3247], and the existence of different water clusters within liquid water [205c, 765, 767g, 767i, 992, 2160], that can rapidly transform between each other and with concentrations that are dependent on temperature and pressure. Generally, two states are hypothesized, although more states may be used. Their coexistence means that these states have identical chemical potential but may differ in their entropy and enthalpy, with these differences being balanced. Such clusters are either low-density (low entropy but high enthalpy), similar in density to hexagonal ice) or of higher density (high entropy but low enthalpy). Also, the surface interactions of such clusters with other molecules/clusters have to be taken into account. Their fluctuating relative concentrations determine the changes in the physical properties. These structural forms must exhibit a relatively large difference in volume between the low-density open and the high-density close-packed structures, if they are to explain the anomalous properties of water. Thus the properties of water are shown to follow those of a more typical liquid at higher temperatures (> 50 °C) based on the higher density form and follow those of a very different liquid at lower temperatures (< 0 °C) based on the lower density form, with about equal amounts of the two forms present at about 0 °C. Such two-state theories have been proposed for well over 100 years. Throughout that time they have been successfully used to explain the anomalous properties of liquid water [23, 24, 25, 56, 57, 262, 268, 276, 409, 699, 826, 1150, 1334, 1353, 1354, 1588, 1595, 1603, 1604, 1612, 1639, 1640, 1674, 1691, 1738, 1757, 1763, 1859, 1909, 1980, 1996, 2019, 2051, 2089, 2127, 2129, 2144, 2189, 2295, 2341, 2505, 2569, 2602, 2653, 2658, 2727, 2755, 2794, 2890, 2918, 2925, 2930, 2972, 3014, 3086, 3111, 3122, 3162, 3203, 3238, 3284, 3296, 3317, 3355, 3358, 3363, 3455b, 3503, 3607, 3635, 3654, 3660, 3696, 3851, 3959, 4000, 4054, 4076, 4094, 4122, 4461]. The main steps in the progress of two-state proposals for water are given below.
Low-density ice-like particles
Higher-density liquid matrix
The first reported suggestion for molecular clusters being responsible for water's anomalous density maximum was by Whiting in 1884 and Röntgen in 1892 [764]. Melting ice was proposed to release solid low-density ice crystals (left) which remained within the higher density matrix (right). Whiting proposed that the concentrations of these solid particles change with temperature and pressure. Chadwell [765] reviewed the first 40 years in the development of this idea.
Bousfield and Lowry [767f] described water as a mixture of less-dense single-molecules (H2O, like steam) and higher-density dimers (H2O)2 (called dihydrol) to explain water's interaction with hydroxide ions. Later, trimers (H2O)3 (called trihydrol) and ice crystallites were added to the proposed system to help describe pure water and the changes towards ice formation. Although this system successfully explainied some properties of water, its popularity dropped when more information concerning the structuring of water became available and the additional clusters needed to fit the density data better. Armstrong [767k] described water as an equilibrium (H2O)n = n(H2O) where n may have several values. These extensions to the hypothesis were described in the Meeting of the Faraday Society held in 1910 [767g].

Low-density ice-like clusters
![]()
Higher-density clusters
In 1933, Bernal and Fowler developed this model in two icrucial respects [1177]. Firstly the denser matrix was proposed to be made up of quartz-like clusters (right) that may close-pack together. Secondly, and significantly, they introduced the idea of equilibration of water molecules interconverting between the clusters [766a], but as the temperature is raised rather than always coexisting. The less-dense structure remained ice-like (see far left) and was only significantly present below 4 °C. Above 200 °C, the water behaved like any other close-packed liquid.
This hypothesis was developed by Pople in 1951 [766b] to describe a one-state continuum model involving a continuous angular distortion of hydrogen bonds. Despite it showing poor agreement with the radial distribution function, Pople's paper proved very influential to many physicists until the more recent two-state models were proven.

Low-density ice-like clusters
Cluster plus an interstitial molecule
An interesting idea, based on interstitial water molecules within the ice hexagonal box (right), was put forward by Samoilov in 1946 [767a] and Narten and Levy in 1967 [767d] based on their X-ray data. Such clusters are not now generally thought to be present in significant quantities. However, some researchers still use this hypothesis [2009], as the interstitial structure approximates a close-packed water structure. It has 1.5 times the density of ice as there are twice as many molecules as cavities.
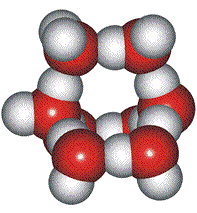
Low-density (H2O)8, clusters
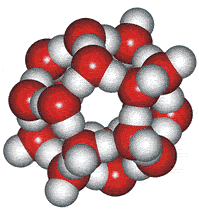
High-density structures
Eucken's theory [767d] was that the molecules in liquid water form an equilibrium between monomers, dimers, tetramers, and octamers. The octamers take up a larger per-molecule volume and occur importantly in many other models, including the icosahedral water cluster model. Eucken suggested that about 30 % of the water molecules are in the octamers. Hall simplified this theory in 1948 [767e] when the high-density structures were less specifically defined.
Frank and Wen [97] described water as a mixture of more-dense single-molecules (H2O) and lower-density hydrogen-bonded clusters (H2O)n. They introduced the term 'flickering structures' to describe the short-lived nature of the structures instead of the more stable structures previously envisioned. This term lives on to the present day. They suggested that the half-life of such clusters be 10−10 -10−11 s, to fit with the dielectric and relaxation time of water, but long enough (102 - 103 times the molecular vibrations) to give it a meaningful existence. Later, Frank and Wen's work was developed into the first statements of the possible coexistence of two liquids of the same substance for liquid water, [767g] followed by [767 l] in 1967.
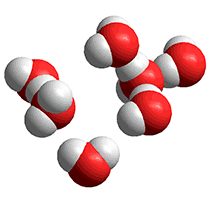
Low-density clathrate clusters
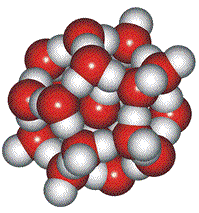
Cluster plus an interstitial molecule
Pauling suggested another interstitial arrangement in 1959, using his interest in clathrate structures [8b]. However, these clusters were quickly dismissed for being unable to explain the available diffraction data if the main constituents in liquid water.
Wada [767h] developed a simplified Eucken model [767d] and described water as a simple two-state model that water is equilibrated between the so-called "iceberg", having a locally low-density ice structure and a more closely-packed high-density structure.
(H2O)icy ![]() (H2O)packed
(H2O)packed
The model gave a 42% composition of the (H2O)icy state at 0 °C. It was used to explain many of the properties of liquid water.
Nemethy and Scheraga [767g] described water as a mixture of more-dense single-molecules (H2O) and lower-density hydrogen-bonded clusters (H2O)n. It was based on Frank and Wen's model [97] and used to help understand the thermodynamic properties of water.

Low-density ice-like particles
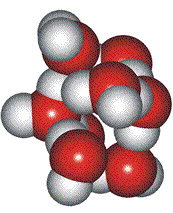
Close-packed hexagons
Davis and Litovitz proposed a variation on Whiting's hypothesis [2160]. The high-density state consists of almost close-packed water hexagon rings, with the low-density state being open ice-like hexagonal boxes. The fraction of ice-like particles was about 0.6 at 0 °C.

Low-density ice-like particles

Higher-density liquid of
unbonded monomers
Arakawa and Sasaki also proposed a variation on Whiting's hypothesis [2161]. The high-density state consists of close-packed unbonded molecules. The fraction of ice-like particles was 0.52 at 0 °C.
Support for the two-state structuring of liquid water also came from the Raman spectral studies of Walrafen in 1968 [4410], and later restated, e.g., [786].
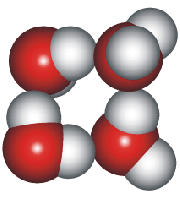
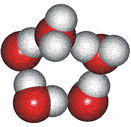
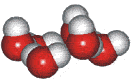
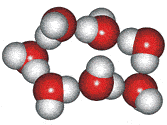
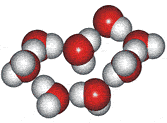
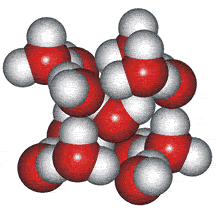
4-, 5-, 6-, 7- and 8-membered rings
A random network model of water, also introduced by Bernal [1177] and published in 1975 [19], contained a mixture of water clusters including 4-, 5-, 6-, 7-, and 8-membered rings. Some success has been had using this model, but its homogeneous nature is not universally applicable or productive.

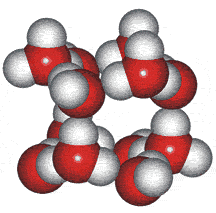
An ice Ih cluster
Wilse Robinson's research group introduced the outer structure two-state mixture model in 1987, b which involved a mixture of water clusters related to ices 1h, II, and III. The group produced several papers using the model to successfully and quantitatively explain many of water's anomalies (for example, [23, 56, 57, 60, 69, 73, 148, 826, 1354]).
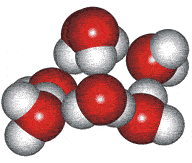
An ice III cluster
The difficulty with this model concerned whether such clusters could exist for significant lengths of time in the liquid water. Therefore, the clusters were more realistically considered as rapidly fluctuating 'indicative' (or representative) structures [1354c].



In 1998 Dougherty and Howard proposed an equilibrium model for water [15] involving dodecahedra, 5- and 6-membered clusters based on several of water's anomalous properties.

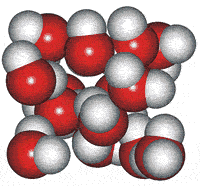
High-density cluster
These water cluster models lead logically to the icosahedral water cluster model, published in 2000 [55] and described at this site. This model is based on dense and less dense clusters equivalent to an equilibrium opposite (see animated gif). The less-dense bicyclo(2,2,2) structures occur when the hydrogen-bonding is strongest, and the dense structures (far left) occur when the weaker but more numerous van der Waals interactions predominate.
![]()
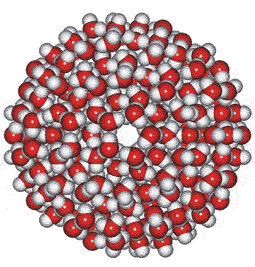
Icosahedral cluster
When conditions arise (such as on supercooling at low temperatures) when there is a high concentration of the expanded 8-membered bicyclo-clusters, (H2O)8, partial to complete icosahedral clusters (right) may arise. Partial structures may involve cyclo-fragments including (H2O)5, (H2O)10, (H2O)12, (H2O)20, and (H2O)100. A full description of this is given elsewhere on this site. These icosahedral clusters contain the dodecahedral, ice Ih cell, 5-, 6-membered and 8-membered bicyclo(2,2,2) subclusters included in the historical survey of models above. This model fits well with and extends the general theory of water two-state clustering.
Later publications have described two-state systems with a low-density state and a high-density state without explicit structures. Partially, this is due to the lack of water monomers found by using vibration spectroscopy. Thus, Maréchal describes them entirely from their infrared spectra [1738], with the low-density form having peaks at 744 cm−1, 1669 cm−1, and 3356 cm−1, whereas the high-density form possesses peaks at 518 cm−1, 1639 cm−1, and 3519 cm−1. Taschin et al. describe them as tetrahedrally hydrogen-bonded, associated with a Raman peak at 225 cm−1, with a disorder associated with a Raman peak at 180 cm−1 [2019]. Finally, Nilsson et al. describe them as tetrahedrally hydrogen-bonded (low-density liquid-like) and closely packed disordered with distorted hydrogen bonds (high-density liquid-like) [1899]. The two states have also been described using the fifth-nearest neighbor, whether it is associated with the inner shell (higher-density water) or the second shell (low-density water) of neighboring water molecules [3203]. This description is substantially similar to other descriptions. A 2020 paper has described the experimental separation of the two states as open tetrahedral structures and local structures lacking tetrahedral symmetry, both in a dynamic equilibrium [3851]. This was also confirmed in ambient liquid water by simulations in 2022 [4461].
Recently (2022), the presence of small amounts (2 - 8%) of a third component has been discussed [4487]. This third component was described as possibly possessing water molecules with five hydrogen-bonds and in clusters significantly denser than either of the main components. These conclusions require further confirmation and this writer believes this component was possibly caused by the interfacial interactions of the main components.
Shows how cluster lifetime is independent
of hydrogen bond lifetime (wait)
The meaning of the term 'cluster' has evolved during this time as well. In the beginning, water clusters were thought of as discrete molecular entities, like crystals. These were proposed to have long (e.g., > seconds) individual lifetimes where the same molecules were involved, within the cluster, throughout the cluster lifetime. Nowadays, we know that molecules may leave or add to clusters a with frequencies that depend on their situation. They obey statistical laws with clusters appearing, evolving, and disappearing with the involvement of physically different molecular constituents (that is, the water molecules come and go, see the cartoon, right). The aqueous environment is heterogeneous with more than one type of environment present and the relative preponderance of these environments changing with temperature, pressure, solutes, and surfaces. Clusters are now thought of as dynamic entities offering a simplified view into a complex, broken, and rapidly shifting environment. As such, they reveal water's underlying, if elusive, nature. Locally favored clusters and their preferred connecting structures possess enthalpy and conformational entropy dependant on the cluster topologies. The interior of the clusters favor both high-density patches and low density hydrophobic holes.. Higher emperatures break up large clusters into smalerl ones [4496]..
[Back to Top ![]() ]
]
There is some confusion over whether water should be considered as one liquid or two. Indeed, liquid water under ambient conditions usually appears to be homogeneous on the macroscopic scale. However, this is not so if it is considered at the nanoscopic scale. Concluding that it is one homogeneous phase, even at the nanoscopic level, is contrary to the evidence concerning its change in behavior at low temperatures, dismisses the widely held second critical point hypothesis, and ignores the experimental findings of the second critical point [2602, 3134, 3202]. However, it is broadcasted by using one-state molecular models, associated averaging scattering-data methodology that is incompatible with situations where two liquids or heterogeneities are present and incapable of correctly forecasting water's properties. c Another homogeneou material on the macroscopic scale but heterogeneous on the nanoscopic scale is aqueous alcohol.
Much recent work revolves around the theory that there is dynamical coexistence of two types of local structures in liquid water, (a) locally favored cooperative tetrahedral structure and (b) disordered 'normal liquid' structure [4122].
[Back to Top ![]() ]
]
a Some authors prefer the term 'dynamic heterogeneities' to 'clusters', but this site does not. The same type of structuring is meant in both cases, although 'dynamic heterogeneities' may include the scenario where there is a gradual change in cluster type with temperature (the type being a single cluster arrangement under a set of conditions) whereas 'cluster' is more easily understood in two-state systems as a mixture of cluster arrangements in equilibrium with each other. [Back]
b
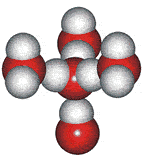
The outer structure two-state mixture model has been successfully used to explain many of the anomalous properties of water (for example, [23, 56, 57, 60, 69, 73, 148, 826, 1354]). Although this model explicitly includes ice Ih (hexagonal ice) and ice-two (ice II) substructures, these may be thought of as representing fully tetrahedral open low-density structures (ice Ih equivalent in the icosahedral network model to ES) and tetrahedral
14-molecule tetrahedron of water molecules
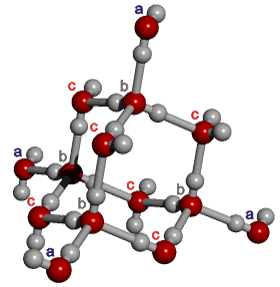
structures with close second neighbors, giving
a higher density 'collapsed' structure (ice-two equivalent in the icosahedral network model to CS).
This equivalence can best be envisioned by examining the behavior
of a 'c'-type water molecule.
They are the most numerous water molecules in icosahedral
water clusters (120/280 = 43%), and they are the least affected
by the movements due to the ES![]() CS equilibrium. These water molecules are tetrahedrally hydrogen-bonded
to the surrounding four (2 'b'
and 2 'c' type) water molecules in both ES and CS.
In ES, the next 'outer' structure (including 2
'a' type) water molecules are also tetrahedrally positioned,. However, in CS the two 'a' type water molecules in this 'outer' structure are flexible and
collapsed, allowing close second neighbors. It may be noted
that the outer structure two-state mixture model does not
depend on the explicit structures of ice Ih and ice-two in order to explain
water's anomalous properties. In fact, the existence of such
explicit structures is unlikely as shown by many of the other
properties of water (for example, see earlier).
This means that arguments using this model can also support the icosahedral cluster
model (but not explicitly vice versa). [Back]
CS equilibrium. These water molecules are tetrahedrally hydrogen-bonded
to the surrounding four (2 'b'
and 2 'c' type) water molecules in both ES and CS.
In ES, the next 'outer' structure (including 2
'a' type) water molecules are also tetrahedrally positioned,. However, in CS the two 'a' type water molecules in this 'outer' structure are flexible and
collapsed, allowing close second neighbors. It may be noted
that the outer structure two-state mixture model does not
depend on the explicit structures of ice Ih and ice-two in order to explain
water's anomalous properties. In fact, the existence of such
explicit structures is unlikely as shown by many of the other
properties of water (for example, see earlier).
This means that arguments using this model can also support the icosahedral cluster
model (but not explicitly vice versa). [Back]
[Back to Top ![]() ]
]
c If such principles were applied to an analysis of Earth's human population, they would only discover and describe one gender of intermediate physiology between man and woman. [Back]
Home | Site Index | Icosahedral water cluster model | LSBU | Top
This page was established in 2005 and last updated by Martin Chaplin on 7 September, 2022