
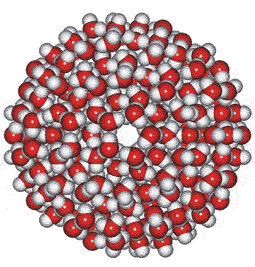
280-Molecule icosahedral open water cluster
![]() Overview of the structure of liquid water
Overview of the structure of liquid water
![]() Introduction to water clustering
Introduction to water clustering
It is reasonable that the structure of liquid water should be related to the structures of hexagonal (1h) and cubic (1c) ices that exist at atmospheric pressure. A further structure for ice (as found in some cubic ice [1236]) is possible by alternating sheets from these boat (from ice Ih) and chair (from ice Ic) water hexamer lattices. Such structures contain 14-molecule tetrahedra (shown below right and further below left).
14-Molecule water tetrahedral cluster
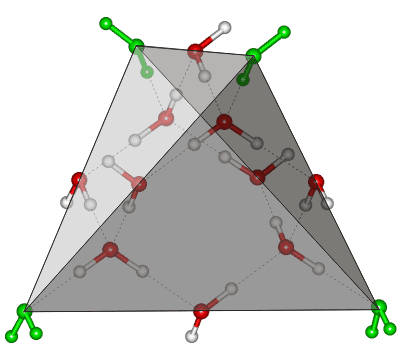
The tetrahedral water cluster, consisting of 14 water molecules, is shown left. There are six water molecules on each face and three on each edge, and four water molecules are internal to the tetrahedron. This cluster occurs in cubic ice and is topologically identical to clusters in a diamondcrystal.
The central ten (shown red) molecules form a strong cluster, and the remaining four (shown green at the vertices) water molecules form pentagons in the completed icosahedral cluster (see below). An earlier structural model for water, also developed (as this one) using X-ray diffraction data, consisted entirely of these ten-molecule tetrahedral clusters (shown red), albeit slightly flattened [398].
For interactive Figures, see Jmol.
14-Molecule tetrahedron of water molecules
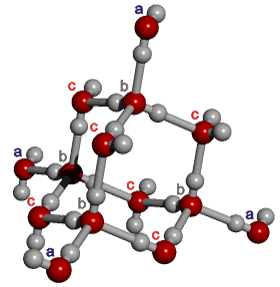
There are three different environments
for the water molecules in these tetrahedra; labeled a, b, and c.
The four water molecules, labeled (a),
form the corners of the tetrahedron and each is involved in
six boat-form hexamers and three pentamers in the icosahedral clusters (see below). These (a) water molecules hydrogen
bond to the four molecules labeled (b),
internal to the tetrahedron, each involved in nine
boat-form and three chair-form hexamers. These (b) water molecules, in turn, hydrogen
bond to the remaining six (c)
molecules of water, positioned midway along each tetrahedral
edge, each involved in one pentamer, eight boat-form,
and two chair-form hexamers. The
central ten water molecules in these units (labeled 'b' and 'c') form an adamantane-type ring structure (tricyclo[3.3.1.13,7]decane),
identical to the ten-molecule unit found in a crystalline
supramolecular complexes [32],
as found within the 18-molecule cubic ice cell (ice Ic structure, ice-seven and ice-eight)
and as also found as an eight-water cyclic cluster substructure
(missing the four 'a'-labeled and two oppositely-positioned
'c'-labeled water molecules but otherwise as shown right) in another supramolecular complex [249]. [Back to Top ![]() ]
]
The regular arrangement of twenty of these 14-molecule structures (albeit utilizing slightly flattened tetrahedral units where three edges are 5% shorter than the other three) may form an icosahedral network. Such clusters appear to be relatively stable in liquid water, forming curved surfaces when bound together using the three potential hydrogen bonds on each of their faces. Twenty of the 14-molecule tetrahedral units, together containing 280 molecules of water, may form a 3 nm diameter a icosahedral structure (see below left), with small differences in geometry throughout b being taken up by the flexibility of the hydrogen-bonding. The icosahedral (H2O)280 water cluster shows increased stabilization as the shells increase in the order (H2O)20 < (H2O)100< (H2O)280 [1619].h
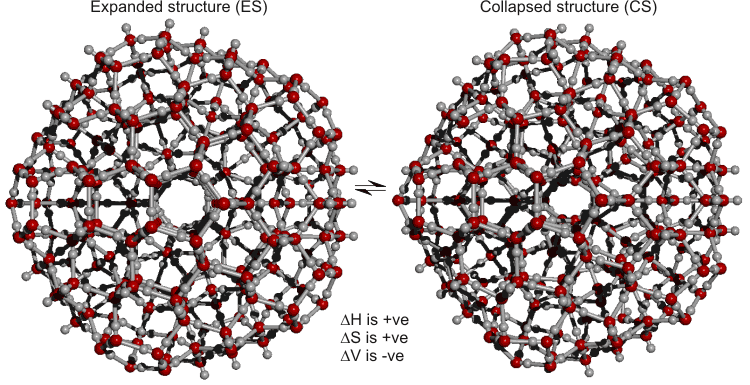
CS. The ES structure collapsed into the puckered central dodecahedron (for interactive Figures, see Jmol), shown separately in substructure g below. The puckering, considered here, is symmetrical with 12 outer positions at 4.15 Å from the center and eight inner ones (arranged at the vertices of a 3.14 Å cube) at 2.71 Å from the center. A different puckering can occur to give, for example, four or six equivalent inner positions.
This icosahedral packing of the tetrahedral units is managed by each of their four tetrahedral chair-form hexameric faces forming three hydrogen bonds to neighboring units, so creating structural units identical to the hexameric boxes present in hexagonal ice (substructure d). Each tetrahedral edge forms the fifth part of two 15-membered pentagonal boxes made up of five boat-form hexamers c (substructure h) and as found 12-fold within a cavity-encapsulated water nanodrop in a polyoxomolybdate [417]. In forming these links, 8-membered structures (Fig. 4f), representative of the structure of hexagonal ice (arranged similarly to the carbon atoms in bicyclo[2.2.2]octane) and each containing three boat-form hexamers c, are formed near the vertices. Such octameric units, to which every one of the 280 molecules in the expanded icosahedral cluster structure (above left) may be considered belonging, have been suggested as the most probable candidate for favored clusters [86] in water. At its vertices, each tetrahedron donates one molecule to the formation of a dodecahedron (substructure. f). A connectivity map (Schlegel diagram) of the icosahedral structure is shown below (also see an icosahedral and a truncated icosahedral paper model). Although such an icosahedral cluster is capable of tessellation, of its constituent 14-molecule tetrahedra, in three dimensions, albeit increasingly strained with increased size [289] (shown elsewhere), it is incapable of forming a crystalline structure due to its five-fold symmetry. The icosahedral structure contains large interstitial cavities that may allow occupancy by suitable solutes.
The equilibrium shown should only be taken as indicative of the many processes involving partial and networked structures
that occur and which change with temperature e in line with Le Chatelier's principle (ES![]() CS as temperature rises). [Back to Top
CS as temperature rises). [Back to Top ![]() ]
]
Each 280-molecule icosahedron contains a variety of substructures, with each water molecule being involved in four hydrogen bonds;
two as donor and two as acceptor. Cyclic pentamers of water have
bond angles of 108°, which are 1.47° closer to the supposedly
most stable H-O-H angle as evident in water vapor (104.52°)
than are the tetrahedral angles (109.47°) in ice, which may
strengthen the hydrogen-bonding that forms the spines of the cluster.
The clusters can tessellate in three dimensions. Each cluster
has twelve potential sites at its icosahedral vertices for use as
centers of neighboring overlapping clusters, showing the ES![]() CS equilibrium. As the network grows, the structure becomes more
distorted. This tessellation is achieved by the
clusters pulsating (ES
CS equilibrium. As the network grows, the structure becomes more
distorted. This tessellation is achieved by the
clusters pulsating (ES![]() CS)
and flickering (a term introduced by Frank and Wen [97]
over 40 years ago, and now understood to be the exchange between a continuum of structures based around two minimum energy basins) between different central dodecahedra giving
statistically equivalent but geometrically different structuring.
This theory is in line with those of both Luck [18],
(who used vibration data to suggest clusters of 240 molecules with
relatively disordered boundaries at room temperature) and Watterson
[546], who suggested
that flickering clusters form standing waves with a (cluster dimension)
half-wavelength of about 3 nm.
CS)
and flickering (a term introduced by Frank and Wen [97]
over 40 years ago, and now understood to be the exchange between a continuum of structures based around two minimum energy basins) between different central dodecahedra giving
statistically equivalent but geometrically different structuring.
This theory is in line with those of both Luck [18],
(who used vibration data to suggest clusters of 240 molecules with
relatively disordered boundaries at room temperature) and Watterson
[546], who suggested
that flickering clusters form standing waves with a (cluster dimension)
half-wavelength of about 3 nm.
Different hydrogen-bonding configurations with different stability [435] and temperature-dependent energetic variations, cause the cluster stability changes that result in collapse or expansion. Such network structures represent the time-averaged positions and are likely to be incomplete at higher temperatures. Additional fluctuations may involve partial and other polytetrahedral [289] clusters similar to that proposed for liquid lead, where clustering fluctuates between partial but ordered (close-packed icosahedral) structures [162]. Complete, if strained or imperfect, tessellation may be possible, as the TIP4P water model has been shown to form infinite 4-coordinated hydrogen-bonded networks in low-density supercooled water [33]. It is possible that infinite ordered and relatively strain-free tessellation can result from a further interesting property of the 14-molecule tetrahedral units; they can also form low distortion clusters around other less-preferred cavities, such as Anick's smaller optimized cavities or the larger 51262 and 51264 cavities found in crystalline gas clathrates. These contain faces where four or six 14-molecule tetrahedral units come together; both structures show greater local distortion than pentameric tetrahedral units, but the presence of hexameric units reduces the overall strain of extended tessellated structures [289]. Whereas larger cavities may occur under some circumstances if stabilized by occupation with suitable guest molecules, tetrameric units cannot be so easily stabilized.
The stability of the network is finely balanced, being able to
fluctuate between an expanded low-density structure (ES,
Fig. 2, left) and a denser collapsed one (CS,
Fig. 2, right) without breaking any hydrogen bonds and consequent
on small changes in the hydrogen bond strength relative to the non-bonded
interactions. There are minimal Gibbs free energy differences between these structures due to the balanced but opposing changes in entropy and enthalpy. Using a kθ of
3.68 kJ ˣ mol−1 rad−2 in the model, the central
dodecahedron is fully expanded, and the standard deviation of the
angles about the tetrahedral angle is 1.3°. Reducing the kθ by 1% to 3.64 kJ ˣ mol−1 rad−2 (here used as
a mechanism to mimic a slight reduction in the relative strength
of the hydrogen-bonding) causes the central dodecahedron to pucker
inwards and increases this standard deviation to 13.6° about
a mean of 108°. The expanded structure (ES,
Fig. 2, left) with central convex
dodecahedra is formed when stronger hydrogen bonds are present,
as shown by [403]d.
This may occur because of the presence of structuring solutes or
surface interactions. If the hydrogen bonds are weaker,
non-bonded interactions are more important and the cluster forms
a partially collapsed structure (CS,
Fig. 2, right) due to the formation of cubic cavity puckered
dodecahedra. As there are five equivalent ways that these puckered
dodecahedra can form, the actual CS structure will be a fluctuating mixture of inter-converting puckered
forms with similar radial distribution functions. [Back to Top ![]() ]
]
The density of ES is 0.94 g cm−3, and that of CS,
1.00 g cm−3. The former may be compared with the density
of low-density water found around macromolecules [4]
at 0.96 g cm−3, supercooled water (-45 °C) at 0.94
g cm−3 (extrapolated from [70]), the density of low-density amorphous ice (LDA) at 0.94 g cm−3 [30, 34] or estimated for the low-temperature form of liquid water from infrared measurements (0.92 g cm−3) [1738],
while the latter compares with the density of water at 0 °C
of 1.00 g cm−3. With appropriate parameters mimicking
weaker hydrogen bonding or greater pressure, CS is capable of further collapse, increasing this density. The CS structure involves the collapse of the central dodecahedron only
out of the four dodecahedra associated with the 280-molecule cluster
(the other three dodecahedra exist as 12 quarter-dodecahedra at
the periphery). The collapse of all four dodecahedral structures would
be expected to increase the density about a further three-fold from
that between the ES and CS structures to gives a density of about 1.18 g cm−3, similar
to that of high-density amorphous ice (1.17
g cm−3, [34]) or that estimated for the high-temperature form of liquid water from infrared measurements (1.12 g cm−3) [1738]. Such collapse
also gives an increase in the closely-approaching 'interstitial'
water, 2-, 3- and 4- hydrogen bonds removed from given water molecules,
as found by molecular dynamics of high-density water [482]. [Back to Top ![]() ]
]
Cluster components
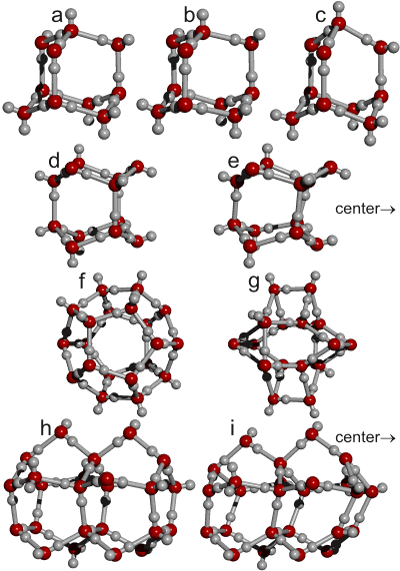
The sub-structures found in the expanded (ES; a, d, f, h) and equivalent forms in the collapsed (CS; b, c, e, g, i) water structure are shown right.
Structure (d, (H2O)12, 1.25 H-bonds/H2O)) shows the hexameric box formed by the faces of the tetrahedral. Structure (f, (H2O)20, 1.5 H-bonds/H2O)) shows the dodecahedron formed by the vertices of the tetrahedra. Structure (h, (H2O)25, 1.0 H-bonds/H2O)) shows the pentagonal box formed by the edges using similar molecules from five tetrahedron edges, meeting at two pentagonal faces. Each tetrahedron unit has a fifth share in each pair of such units that form on each of its six edges. Note that the 10-molecule unit (a, (H2O)10' 1.2 H-bonds/H2O)) shows the least signs of collapse when in the collapsed structure (b, c) (and are therefore likely to be most stable). In contrast, the 20-molecule unit (f, g) shows the most significant change, and consequently the formation and stabilization of the dodecahedron (f) plays a significant role in forming the clusters and the cluster equilibrium. Cluster h has the greatest bond energy (and least strain) of all polyhedral water clusters [3569]. Cluster h is also used in theoretical dipole studies of water [1993].
The pancake-like cluster below, (H2O)25, 1.4 H-bonds/H2O, is stable in vaccuo as calculated using the Restricted Hartree-Fock wave function (RHF) using the 6-31G** basis set. There are 24 of the structures (overlapping) in one 280-molecule expanded icosahedral water cluster.
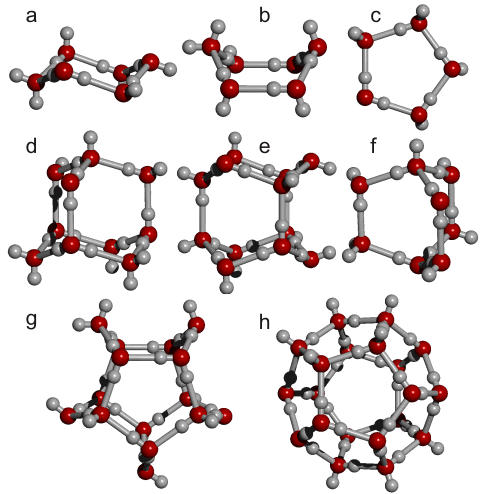
 Only the (H2O)20 dodecahedral structure f and the all-gauche ten-molecule
tetrahedra (d, (H2O)10, below) are also stable in vaccuo as calculated
using the Restricted Hartree-Fock wave function (RHF) using
the 6-31G** basis set out of all the above structures (a - i) above and (d - g) below.
Only the (H2O)20 dodecahedral structure f and the all-gauche ten-molecule
tetrahedra (d, (H2O)10, below) are also stable in vaccuo as calculated
using the Restricted Hartree-Fock wave function (RHF) using
the 6-31G** basis set out of all the above structures (a - i) above and (d - g) below.
The diagram below right illustrates the number of structural forms within the 280-molecule water cluster (ES); the number of type a, b, and c molecules, as described above, is given bracketed below as (na, nb, nc). Interestingly, clusters d, f, g, and h are the (only) four clusters singled out by Stillinger from early molecular dynamics calculations [729] and thought particularly relevant in supercooled water. These clusters form the key to the formation of the 280-molecule water cluster (ES). It is worthy of note that cyclic pentamers (c) and boat-form hexamers (b) appear to be the most stable water pentamer and hexamer structures in the gas phase [466], with cyclic pentamers most likely to remain intact at higher temperatures [731].
In one 280-molecule water cluster (ES) there are:
Structural forms found in the icosahedral water cluster
80 complete all-gauche chair-form hexamers (a, (H2O)6, 1.0 H-bonds/H2O) (0,3,3), f
360 all-gauche boat-form hexamers (b, (H2O)6, 1.0 H-bonds/H2O) (67% 2,2,2 and 33% 0,2,4) of which 90 are made up of partial bits,
72 all-cis pentamers (c, (H2O)5, 1.0 H-bonds/H2O) (5,0,0) of which 36 are made up of partial bits,
20 all-gauche ten-molecule tetrahedra (d, (H2O)10, 1.2 H-bonds/H2O) (0,4,6),
40 all-gauche hexameric boxes (e, (H2O)12, 1.25 H-bonds/H2O) (0,6,6) of which 10 are made up of partial bits,
120 all-gauche eight-molecule structures (f, (H2O)8 1.125 H-bonds/H2O) (2,2,4) of which 30 are made up of partial bits,
48 cis- and gauche-bonded pentameric boxes (g, (H2O)15, 1.33 H-bonds/H2O) (5,5,5) of which 24 are made up of partial bits, and
four all-cis dodecahedra (h, (H2O)20, 1.5 H-bonds/H2O) (20,0,0), of which three are made up of partial bits (that is,12 quarter-dodecahedra)
[Back to Top ![]() ]
]
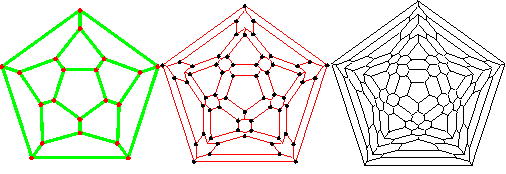
Connectivity diagram of an icosahedral cluster
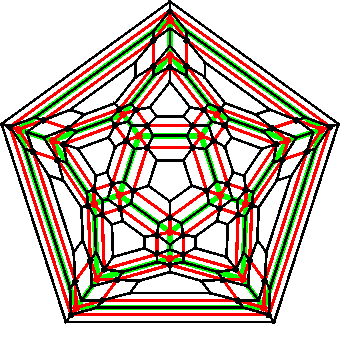
The two-dimensional connectivity map (Schlegel diagram) of the 280-molecule icosahedral clusters is shown right, with the inner (green) middle (red) and outer (black) layers indicated by their color. Each intersection represents one water molecule connected to others by hydrogen bonds. The Schlegel diagrams represent planar graphs reflecting the topology of the
structures.
The inner, middle and outer shells are also shown separately below.
[Back to Top ![]() ]
]
Below left is a Java applet showing the solid shape of the proposed water (H2O)100 inner icosahedral cluster. It is a truncated icosahedron with 12 pentagonal faces (with edge length (el ) of about 0.28 nm), 20 equilateral triangular faces (with edge length of about 2 ˣ (2/3)½ ˣ el; ≈ 0.47 nm) and 30 rectangular faces (with edge lengths of about el and 2 ˣ (2/3)½ ˣ el ). The stability of the (H2O)100 cluster has been confirmed from quantum-chemical computations [1627]. Below right is a Java applet showing the solid shape of the proposed complete water (H2O)280 icosahedral cluster. It is also a truncated icosahedron with twelve pentagonal faces (with edge length ( el ) of about 0.28 nm), 20 equilateral triangular faces (with edge length of about 4 ˣ (2/3)½ ˣ el; ≈ 0.94 nm) and 30 rectangular faces (with edge lengths of about el and 4 ˣ (2/3)½ ˣ el ). The (H2O)100 cluster lies inside the (H2O)280 icosahedral cluster. Centrally inside the (H2O)100 cluster lies an (H2O)20 dodecahedron with (just) twelve pentagonal faces with edge length ( el ). For further interactive Figures, see Jmol (equilibria) and Jmol ((H2O)100 and (H2O)280).
(H2O)100 inner cluster (H2O)280 complete cluster
a The radius is equal to the limit of the order noted in supercooled heavy water [221]. [Back]
b The dihedral angle of a tetrahedron is sin−1(2√2/3) = 70.529°, just short of the 72° required for a perfect fit into an icosahedron. [Back]
c Boat-form water hexamers appear to be the most stable water hexamer structures in the gas phase [466]. [Back]
d About 7% average loss of hydrogen bond strength for unoptimized clusters. [Back]
e The proportion of ES-like water has been estimated at 47% at 0 °C [1354]. Estimates for the thermodynamic changes for water molecules within the cluster can be made based on related 2-state models giving ΔH, ΔS, ΔV of ≈ +1 kJ ˣ mol−1, ≈ +3 J deg−1 mol−1, -1.08 cm3 mol−1 [440], or +1.3 kJ ˣ mol−1, +5.5 J deg−1 mol−1, -2.0 cm3 mol−1 [600], or +1.8 kJ ˣ mol−1 (ΔH, [1353]), or the very different +11.8 kJ ˣ mol−1 (ΔH), +40 J deg−1 mol−1 (ΔS) -3.5 cm3 mol−1 (ΔV) [1738]). [Back]
f Bond torsional angles around the hydrogen bond are known as (i) cis when almost planar, (ii) gauche when about 60° or (iii) trans when about 180°. Cis-hydrogen bonding allows a favorable overlap of the molecular orbitals [165] but is not significantly sterically destabilized relative to the gauche structures as they might be in alkanes (for example). [Back]
g This uses a non-commercial Java 1.1 applet by Martin Kraus. Use the mouse to rotate the structures. [Back]
Fat sheet made from (H2O)12 partial dodecahedra
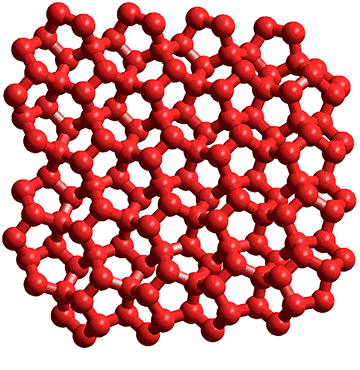
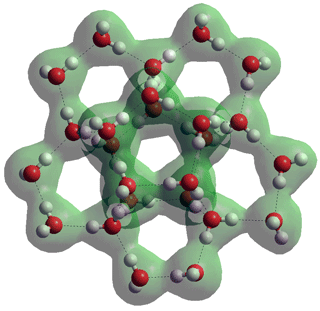
h Water (H2O)20 dodecahedra [1652] and (H2O)100 icosahedra [417] have been found in crystal structures, as has a sheet-like structure formed from water (H2O)12 partial dodecahedra (shown right, slightly twisted with just the oxygen atoms) [1871]. Surprisingly, such sheet structures also occur (without any clathrate inclusions) as the xz sheets in ice_xvii [2796]. [Back]
Home | Site Index | Back: Water: Introduction | Methods for investigating clusters | Brief history of water clusters | LSBU | Top
This page was established in 2000 and last updated by Martin Chaplin on 4 October, 2021