
![]() Water has an unusually high melting
point
Water has an unusually high melting
point
![]() Water has an unusually high boiling
point
Water has an unusually high boiling
point
![]() Water has an unusually high critical
point
Water has an unusually high critical
point
![]() Solid water exists
in a wider variety of stable (and metastable) crystal and
amorphous structures than other materials
Solid water exists
in a wider variety of stable (and metastable) crystal and
amorphous structures than other materials
![]() The thermal conductivity
of ice reduces with increasing pressure
The thermal conductivity
of ice reduces with increasing pressure
![]() The structure of liquid water changes at high pressure
The structure of liquid water changes at high pressure
![]() Supercooled water has two phases and a second critical point at about -91 °C
Supercooled water has two phases and a second critical point at about -91 °C
![]() Liquid water is easily supercooled but glassified with difficulty
Liquid water is easily supercooled but glassified with difficulty
![]() Liquid water exists at very low temperatures and freezes on heating
Liquid water exists at very low temperatures and freezes on heating
![]() Liquid water may be easily superheated
Liquid water may be easily superheated
![]() Hot water may freeze faster than cold
water; the Mpemba effect
Hot water may freeze faster than cold
water; the Mpemba effect
![]() Warm water vibrates longer than cold
water
Warm water vibrates longer than cold
water
![]() Water molecules shrink as the temperature rises
and expand as the pressure increases
Water molecules shrink as the temperature rises
and expand as the pressure increases
![]() A liquid-liquid transition occurs at about 330 K.
A liquid-liquid transition occurs at about 330 K.
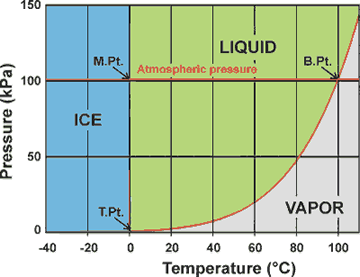
Phase diagram of water showing the melting,
boiling and triple points a
Melting point comparisons
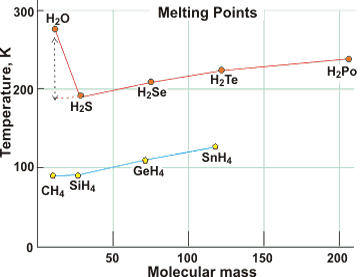
The melting point of water is over 100 K higher than expected by extrapolation of the melting points of other Group 6A hydrides, here above right shown compared with Group 4A hydrides. e It is also much higher than O2 (54 K) or H2 (4 K). See also below for further comparisons.
In ice (Ih), all water molecules participate in four hydrogen bonds (two as donor and two as acceptor) and are held relatively static. Some of the weaker hydrogen bonds must be broken in liquid water to allow the molecules to move around. The considerable energy required for breaking these bonds must be supplied during the melting process. Only a relatively minor amount of energy is reclaimed from the volume change (PΔV = -0.166 J mol−1). The free energy change (ΔG=ΔH-TΔS, where ΔH=ΔU+PΔV) must be zero at the melting point.
As the temperature increases, hydrogen bonding in liquid water decreases, and its entropy increases. Melting will only occur when there is sufficient change in the entropy term (-TΔS) to provide the energy required for the bond breaking (ΔH). The low entropy (high organization) of liquid water causes this melting point to be high.
The melting temperature (Tm ) = ΔHm/ ΔSm = 6006 J ˣ mol−1/22 J ˣ mol−1 ˣ K−1 = 273 K
Interestingly, the equilibrium melting point is reduced by a factor of about '100/diameter (nm)' for water in hydrophilic pores [2236], so allowing very low melting points to be achieved. However, the other properties of such material cannot be equated to those of normal unrestrained 'bulk' water.
Although ice is very difficult to superheat above its (equilibrium) melting point, tiny
amounts of ice (Ih) have been superheated
to 290 K (without melting) for very short periods (>250
ps) [954a] with the limit of superheating (>1 ns) established at about 330 K [954b].
[![]() Anomalies page: Back to Top
Anomalies page: Back to Top ![]() ]
]
Boiling point comparisons at 101.325 kPa
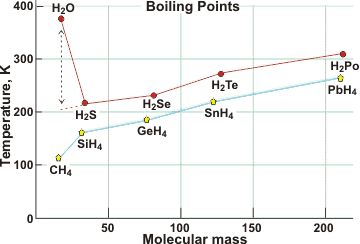
The boiling point of water (100 °C at 101.325 kPa; the usual boiling point) is over 150 K higher than expected by extrapolation of the boiling points of other Group 6A hydrides, here shown compared with Group 4A hydrides. e It is also much higher than O2 (90 K) or H2 (20 K). See also below for further comparisons. The boiling point drops with pressure being only ≈ 72 °C at the summit of Mount Everest (8848 m above mean sea level, ≈ 33.7 kPa) [3065]. The boiling temperature is 99.65 °C at the IUPAC standard pressure of 100 kPa.
There is considerable hydrogen bonding in liquid water, resulting in high cohesion (water's cohesive energy density is 2.6 times that of methanol), which prevents water molecules from being quickly released from the water's surface. Consequentially, the vapor pressure is reduced (water has low vapor pressure). As boiling cannot occur until this vapor pressure equals the external pressure (see graph), a higher temperature is required.
Comparison of boiling and melting points of water
with short-chain alcohols
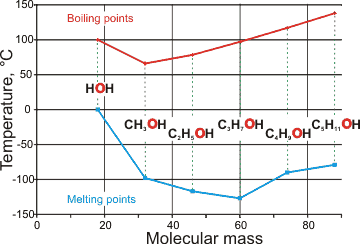
As with the melting point, the boiling temperature,
Tb = ΔHb/ ΔSb
= 40657 J ˣ mol−1/108.95 J ˣ mol−1 ˣ K−1 = 373 K
The high boiling and melting points of water are also evident when compared with the hydrogen-bonding of short-chain alcohols (see right)
The pressure/temperature range of liquidity for water is much larger than for most other materials (for example, under ambient pressure, the liquid range of water is 100 �C whereas, for both H2S and H2Se, it is about 25 �C.
[![]() Anomalies page: Back to Top
Anomalies page: Back to Top ![]() ]
]
Critical point comparisons
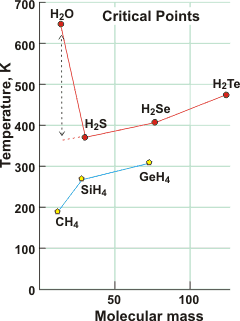
The critical point of water is over 250 K higher than expected by extrapolation of the critical points of other Group 6A hydrides, here shown compared with Group 4A hydrides. e For example, the critical point (647 K, 22.06 MPa 322 kg m−3) is far higher than ethanol (514 K, 6.14 MPa 276 kg m−3), which also hydrogen bonds (but in chains not 3-dimensional) and is much larger and more massive.
The critical point can only be reached when the interactions between the water molecules fall below a certain threshold level. Due to the strength and extent of the hydrogen bonding, much energy is needed to cause such a reduction in molecular interaction, and this necessitates higher temperatures. Even close to the critical point, many hydrogen bonds remain, albeit bent, elongated, and no longer tetrahedrally arranged [92].
Physical properties of isoelectronic molecules
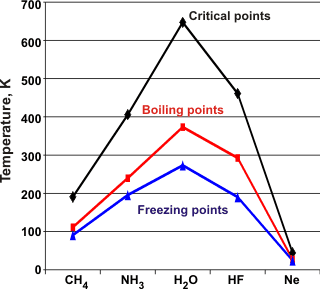
The critical points (C.Pt.), boiling points (B.Pt.), and melting points (M.Pt.) of the molecules isoelectronic with water show water to have higher values.
Ammonia
and hydrogen fluoride also have somewhat raised values. They form molecular clustering, albeit with three donor
H-atoms and one lone pair acceptor group or one donor
H-atom and three lone pair acceptor groups, respectively;
giving a maximum of two hydrogen bonds per molecule, on average. Although
solid HF forms stronger hydrogen bonds, these form linear zigzag chains with no
rings or polygons, and hence its three-dimensional structure
is weaker. The hydrogen bonds in solid NH3 can form three-dimensional arrangements but are distorted and weakened.
Water has two donor H-atoms and two lone pair acceptor
groups with close to tetrahedral angles giving the possibility
of four hydrogen bonds per molecule with little distortion.
[![]() Anomalies page: Back to Top
Anomalies page: Back to Top ![]() ]
]
The ability for water to form extensive networks of hydrogen bonds increases the number of solid phases possible to over fifteen. Both open and close-packed structures are possible. The open structure of hexagonal ice (19.65 cm3 mol−1), which contains only about 7.5 cm3 mol−1 of water molecules, gives plenty of scope for different arrangements of the water molecules as the structure is compressed. For comparison, hydrogen sulfide has only four distinct solid phases [119]. The most unusual phases of ice are, perhaps, ice XVIII and ice XX; black ices, stable at extremely hot temperatures close to that of the surface of the Sun.
Another anomaly is found as the ices change their crystalline phase. Thus there are decreases in volume when ice Ih changes to ice II (ΔV = 3.92 cm3 mol−1), ice II changes to ice V (ΔV = 0.7 cm3 mol−1), ice V changes to ice VI (ΔV = 0.7 cm3 mol−1), and ice VI changes to ice VII (ΔV = 1.05 cm3 mol−1) . However, in all cases, this is associated with increases in internal lattice energy (79 J mol−1, 1.45 kJ ˣ mol−1, 423 J mol−1, 2.3 kJ ˣ mol−1 respectively). Usually, an increase in density is expected to increase the interaction between the molecules and make the internal energy more negative. These phenomena are primarily due to the changes in the hydrogen-bonding strength caused by the molecular rearrangement. [![]() Anomalies page: Back to Top
Anomalies page: Back to Top ![]() ]
]
Hexagonal ice shows anomalous reduction in thermal conductivity,
shear
modulus, and transverse sound velocity with increasing pressure (as do cubic ice and low-density amorphous ice but not high-density amorphous ice ), which
behavior is different from most crystals where these properties increase with increasing density. Low-density amorphous ice is the only glass to show this peculiar behavior. These anomalies are due to the pressure-induced bending of the hydrogen bonding
[617]. The same anomalies are found in D2O hexagonal ice, but to a much lesser extent [1796], showing unexpectedly large isotopic effects due to the greater degree of ordering in D2O hexagonal ice. [![]() Anomalies page: Back to Top
Anomalies page: Back to Top ![]() ]
]
In a similar manner to the formation of the high-density crystalline (ice-five and ice
seven) and amorphous (HDA) ice phases, liquid water also undergoes a significant
change in structure at high pressure (about 200 MPa).
The viscosity, self-diffusion, compressibility, and structural properties of water all change above about 200 MPa. At higher temperatures, these changes occur at higher pressures (400 K at about 600 MPa and 450 K at about 1 GPa) [2758]. Other properties also change around 200 MPa, such as the loss of the density maximum, the discontinuity in fast sound in liquid water, and water molecules moving further away from each other. The explanation for all these effects is that there appears
to be an increase in the interpenetration of hydrogen-bonded networks
at about 200 MPa (at 290 K); interpenetration of hydrogen-bonded clusters being preferred over more extreme bending
or breaking of the hydrogen bonds. This structuring for liquid water at high pressures is consistent with that found by neutron scattering [1001]. It indicates that liquid water structuring at high pressure has similarities to that of its high-pressure ice phases [1254, 2194]. [![]() Anomalies page: Back to Top
Anomalies page: Back to Top ![]() ]
]
Water below its thermodynamic melting point is 'supercooled'. The thermodynamic properties of supercooled water have been reviewed [1794]. The structure of bulk liquid supercooled water below homogeneous nucleation temperature (232 K) has been determined down to 222 K using oxygen K-edge x-ray emission spectroscopy (XES) on rapidly evaporatively cooled micron-sized droplets [2419]. The most likely conclusion drawn from this study is that there are fluctuations between the LDL-like and HDL-like regions, but their ratio remained unclear [2419]. As water is supercooled, it converts mainly into its expanded form (for example, ES) at ambient pressures, which at low enough temperatures (< -38 °C) may result in it forming metastable low-density amorphous ice (LDA; although it usually will form hexagonal ice at this temperature). If the pressure on LDA is increased above about 200 MPa, then LDA undergoes a 30% collapse forming metastable high-density amorphous ice (HDA) but notably in a continuous process without (apparently) breaking the hydrogen bonds [394]. This phase change cannot continue to higher temperatures (so creating a second critical point, [45, 1530, 1888,2029])d as neither of these phases is stable in the presence of liquid water, although they may convert into their metastable supercooled liquid forms. The presence of these low- and higher-density forms of liquid (supercooled) water leads to the breakdown of the Stokes-Einstein relationship in supercooled water [1040], occurring far above the glass-transition temperature, in contrast to many supercooled liquids where this behavior is found only at temperatures just above this transition [1040b].
It is now generally recognized that liquid water above 0°C also consists of smaller clusters of two different states of water, related to LDA and HDA, which may interconvert. [![]() Anomalies page: Back to Top
Anomalies page: Back to Top ![]() ]
]
Water freezing is not merely the reverse of ice melting [1110]. Melting is a single-step process that occurs at the melting point as ice is heated. In contrast, freezing of liquid water on cooling involves ice crystal nucleation and crystal growth that generally is initiated a few degrees below the melting point even for pure water. Liquid water below its melting point is supercooled water. The directional hydrogen-bonding capacity of water reduces its tendency to supercool as it encourages the regular structuring in cold liquid that may lead to a crystalline state. Also, supercooling is more likely in molecules with complex structures, having difficulty fitting into their crystalline lattice.
Effect of water activity on ice melting point, from [457b]
![Effect of water activity on ice melting point, from [457b]](images/activity-ice.gif)
Liquid water, however, is easily supercooled c down to about -25 °C and with more difficulty down to about -38 °C with further supercooling possible, in tiny droplets (≈ 9 μm diameter) down to about -46 °C for short periods (≈ ms) [2143], f and -92 °C at 200 MPa. An increase in salt concentrations up to 2% increases the average freezing point of distilled water from -8 °C to around -2 °C [2873]. Supercooling increases with increasing cooling rate and decreasing volume. Below these temperatures, supercooled water completely loses its stability (it is in a 'no-mans land' where liquid water cannot exist ), and crystal formation rapidly occurs by merging countless small nuclei distributed uniformly over the phase. Water, supercooled down to -37.5 °C, is sustained in storm clouds and the condensed clouds formed by aircraft at high altitudes. Somewhat strangely, at the limit of this supercooling (also known as the homogeneous freezing point), the water activity is always 0.305 higher than that of water melting at the same temperature [457] (see right). Where salts or hydrophilic solutes are present, the homogeneous freezing point is lowered about twice as much as the melting point lowering [663].
Liquid water may be maximally supercooled (Ts) to about -92 °C and 210 MPa. This amount of supercooling (about a third of the melting point (Tm, 273 K) is typical for molecular liquids. However, at normal pressures, the achievable supercooling is much smaller (Ts/Tm ≈ 0.15) due to the effect of the second critical point. It should be noted that bulk water never forms a glass as its glass transition temperature (Tg, = ≈ 136 K) is far lower, relative to its melting point, than expected; Tg/Tm ≈ ½ rather thanTg/Tm ≈ ⅔ as for more typical liquids [4145]. Thus supercooled bulk water (i.e., not affected by surfaces or solutes) always crystallizes before its temperature can be sufficiently lowered, whatever the cooling rate [558]. Water glass may only be produced by extremely rapid cooling (105 K ˣ s−1) of tiny volumes of water (<≈ 100 μm diameter). The glass transition temperature of D2O is ≈ 10 K warmer than H2O, which is an anomalously large difference compared with other hydrogen-bonded liquids that show only ≈ 1 K difference upon H/D substitution [2267].
As water is cooled, the cluster equilibrium shifts towards the more open structure (e.g., ES ) with higher viscosity. For crystallization to occur, at least, 3 - 4 unit cells worth of water molecules have to come together in the correct orientation.b The formation of whole or part icosahedral clusters interferes with this process while not allowing cluster crystallization due to their fivefold symmetry. Such geometric frustration as a mechanism to explain supercooling has been established for some time [2869]. Lowering the temperature further, which should encourage crystallization, is partially counteracted by increased icosahedral clustering. The presence of ES clusters is, in principle, in agreement with computer simulation studies requiring the presence of metastable states [216]. Methods that break the hydrogen bonding in these clusters, such as ultrasonics [296], cause the supercooled water to freeze immediately.
Surprisingly, the glass transition temperatures in dilute aqueous solutions of simple salts (LiCl, LiBr, LiI, NaCl, CsCl MgCl2) and hydrophilic solutes (ethylene glycol, propylene glycol, glycerol) decrease (from ~136 K) on the initial addition of the solute, reach a solute-specific minimum value (~129 K), and then increase again [3754]. This indicates the competing effects of an initially dominant solute-loosening of the H-bond network followed, at higher concentrations, by increased hydrogen-bonding with solutes, hydration of ions, and stability of ion pairs.
There are earlier [569] and more recent [1794] comprehensive reviews of the properties of supercooled
water.
[![]() Anomalies page: Back to Top
Anomalies page: Back to Top ![]() ]
]
Deeply supercooled liquid water can be produced from glassy amorphous ice between
-123 °C and - 149 °C [74]
and may coexist with cubic ice up to -63 °C [137].
This behavior is particularly anomalous as the liquid
(deeply supercooled water) is a 'strong'
liquid (compared with 'normal' supercooled water that is a 'fragile'
liquid [493]) that
changes to crystalline solid (cubic ice)
on increasing the temperature while keeping the pressure
constant. Such a 'strong'
to 'fragile'
(Arrhenius to non-Arrhenius) change in a liquid is not normal. Deeply supercooled water exists in the liquid state where it appears to be too cold to diffuse sufficiently quickly to crystallize noticeably. A possible explanation of this low-temperature-range liquid water
may be the formation of strands of icosahedral
structures. This model can also explain the high viscosity and strong (that is, low specific heat) liquid behavior of this
extremely supercooled water [215]. The unusual behavior of this liquid (that is, deeply supercooled water), by solidifying on heating, has been found with other liquids (for example, methyl-cellulose and some cyclodextrin solutions [1026]).
[![]() Anomalies page: Back to Top
Anomalies page: Back to Top ![]() ]
]
Liquid water can be easily superheated above its boiling point away from its surface with the atmosphere [1128, 1184] (as originally described by Michael Faraday in 1850 [4467]). This may be particularly important when heating foods and drinks in a microwave oven where explosive steam production from the superheated water may cause severe injuries. Superheating also causes the boiling point of water to vary, in much the same way as its freezing point, and of irregular boiling, that is, 'bumping' [1184]. Liquid water may be superheated at one atmosphere to about +240 °C to +280 °C in capillaries or tiny droplets within high-boiling immiscible solvents (the limit of superheating, also called the spinodal temperature, is about 330 °C [1646]). Degassing water increases its tendency to superheat [1825]. The use of “boiling chips” (mm-sized glass granules) avoids superheating and facilitates smooth boiling.
Superheating is also apparent at low temperatures but negative pressures (i.e., stretched water). Water may be superheated by reducing the pressure to below -100 MPa at 20 °C [1128]. Superheating is facilitated by dissolved gas that may increase its hydrogen-bonded order [821]. However, it is prevented by the presence of gas bubbles or nanobubbles (that is, cavities) that act as initiation sites for vaporization.
Water vapor (gas) may easily be supercooled below its condensation temperature (dew point) for its partial pressure (i.e., its boiling point ) in the absence of dust or other particles or surfaces that help the nucleation process [1184].
[![]() Anomalies page: Back to Top
Anomalies page: Back to Top ![]() ]
]
It is expected that the lifetime of an excited molecular vibration should decrease as the temperature increases as the energy and likelihood of interactions with other molecules also increase. For example, the lifetime of the excited liquid HCl stretch vibration decreases from 2.1 ns at 173 K to 1.0 ns at 248 K.
In liquid water, the excited OH-stretch vibration has a lifetime
of 0.26 ps at 298 K, and this lifetime increases to 0.32 ps at 358 K [592].
The reason for this is due to the effects of the hydrogen-bonded
network. The OH-stretch vibration relaxes typically by transferring
energy to an overtone of the H-O-H bending vibration. However, as
the temperature increases, the hydrogen bonds of water get weaker,
which leads to an increase in the frequency of the stretch vibration
and a decrease of the frequency of the bending vibration. As a result,
the overtone of the bending mode shifts out of resonance with the
stretching mode, thereby making the energy transfer less likely.
[![]() Anomalies page: Back to Top
Anomalies page: Back to Top ![]() ]
]
Changes in the H-bond partial bond lengths, from [2044]
![Changes in the H-bond partial bond lengths with increasing temperature: from [2044] Changes in the H-bond partial bond lengths with increasing temperature: from [2044]](images/h_bond_temperature.gif)
It is expected that molecular bond lengths will increase, and bonds will bend more as the temperature increases, giving rise to slight increases in the volume of individual molecules. However, as the temperature rises (> 4 °C), the water molecules move away from each other (shown blue right, upper curve), and the hydrogen bonds weaken (shown green dotted right, middle curve). This causes the O-H covalent bonds (shown red right, lower curve) to shrink (the O-H bond length is 97.99 pm at 4 °C but 97.86 pm at 50 °C), strengthen and stiffen, so reducing the volume of individual water molecules [2044]. Thus, the water molecules shrink as the temperature rises.
Covalent and H-bond lengths in ice under pressure, from [1809]
![variation in covalent and H-bond lengths in ice under pressure [1809] variation in covalent and H-bond lengths in ice under pressure [1809]](images/h_bond_pressure.gif)
As covalent bonds generally shorten under high pressure, a further anomalous change is that the O-H covalent bond length of water (shown red left, lower curve), in the liquid and solid phases, increases and weakens as the pressure increases (the O-H bond length is 97.98 pm at 0.1 MPa but 101.2 pm at 60 GPa). This is due to the hydrogen bond shortening under pressure (shown green dotted left, middle curve). Thus the water molecules expand as the pressure rises.
The variation of bond lengths with pressure for hexagonal ice is shown above left [1809]. A similar effect is expected for ice VII [2538].
[![]() Anomalies page: Back to Top
Anomalies page: Back to Top ![]() ]
]
Brownian motion of nanoparticles in water
from [4042]
![Brownian motion of nanoparticles in water
from [4042] Brownian motion of nanoparticles in water](images/perc.gif)
There is a phase transition where the low-density-liquid (LDL) phase becomes percolated (i.e., forms long-range connectivity throughout the solution) below 330 K [4042]. This transition is evident from the temperature-dependence of the instantaneous Brownian velocities of the 24 nm NaYF4:Yb/Er nanoparticles (see right), and 106 nm nanoparticles in water suspensions, the effective diffusivity of the nanofluid with 24 nm nanoparticles (pH = 5.10) with respect to pure water, and the dependence on the pH of the crossover temperatures.
Around this critical temperature, the sizes of the LDL clusters were similar to the nanoparticles.
[![]() Anomalies page: Back to Top
Anomalies page: Back to Top ![]() ]
]
a The surface temperature on Mars lies below the triple point of water, and its atmospheric pressure is close to this value, such that no liquid water may be found there. [Back]
b Theoretical considerations concerning the ice nucleation site size give estimates of 45,000 water molecules at -5 °C down to 70 water molecules at -40 °C [265]. Molecular dynamics studies show that these do not need to form a crystalline structure for crystallization to occur [347]. [Back]
c The ease of supercooling water was noted by Daniel Gabriel Fahrenheit (1686-1736), who chose his temperature zero-point using a mixture of water, ice, and sal ammoniac (mineral NH4Cl) and the lowest temperature that he could supercool water (0 °F, ≈ 255 K). Supercooling of liquids varies and is measured by the difference between the melting temperature Tm and the homogeneous nucleation temperature TH, normalized by Tm. The ratio (Tm − TH)/Tm is usually about 0.3, but its value for liquid water is 0.154 (Tm = 273 K, TH = 231 K) with a maximum of 0.34 under 200 MPa pressure [4063].
It has been claimed that classical nucleation theory shows that bulk water freezing does not occur at temperatures above -30 °C, and that at higher temperatures ice nucleation requires the presence of some ice-binding surfaces [4482]. [Back]
d There is still some debate over whether this second critical point exists; [2150], but this reference concentrates only on ambient pressure data. This site takes the view that the second critical point does exist. [Back]
e Further related data has been tabulated. [Back]
f The value of -46 °C has been disputed, with the actual value being slightly warmer [2993]. [Back]
![]() Density anomalies (D1-D22) explanations
Density anomalies (D1-D22) explanations
![]() Material anomalies(M1-M16) explanations
Material anomalies(M1-M16) explanations
![]() Thermodynamic anomalies (T1-T11) explanations
Thermodynamic anomalies (T1-T11) explanations
![]() Physical anomalies (F1-F10) explanations
Physical anomalies (F1-F10) explanations
Home | Site Index | The anomalies of water | Water: Introduction | The icosahedral water clusters | LSBU | Top
This page was established in 2006 and last updated by Martin Chaplin on 24 August, 2022