
Ice-XV; 2 hexameric clusters are shown with their 6 further links
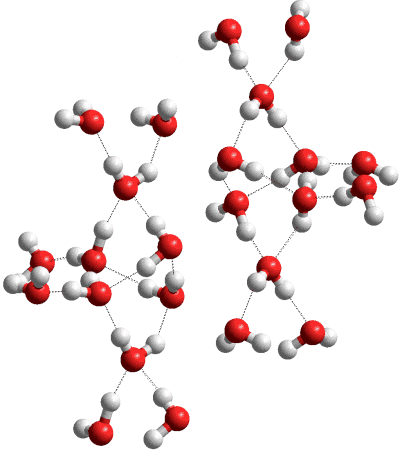
Ice XV is a slightly expanded hydrogen-bonding ordered form of the ice VI phase. Its existence was surmised based on the presence of the ordered forms of other ices at low temperatures. The structure of the fully deuterated form (D2O) was first described in 2009 [1582]. It was prepared by using 10 mM DCl (deuterated hydrochloric acid, about one molecule to every 5000 molecules of water) as a dopant to help the ordering rearrangement of the hydrogen bonding at low temperatures (<130 K). Other dopants have been tried [3324]. Hydrogen ordering is faster in H2O (using HCl) ice VI than in D2O ice VI, with the process possibly transitioning through ferroelectric intermediates [2371].
Ice XV is antiferroelectric, with all the water molecule dipoles canceling out as each of the two separate interpenetrating networks possess oppositely aligned hydrogen bonding. As with ice VIII, other ordered but ferroelectric hydrogen-bonding is possible, but such crystals have not yet been found.
The pseudo-orthorhombic
crystal (shown opposite), is in ![]() space group, has cell dimensions a = 6.2323 Å, b = 6.2438 Å, c = 5.7903 Å (90.06º, 89.99º, 89.92º;
D2O, at ≈ 0.9 GPa and 80 K) [1582]. During the ordering of the hydrogen-bonding of ice VI, the a and b lattice constants contract, whereas there is a pronounced expansion in the c lattice constant [2687].
space group, has cell dimensions a = 6.2323 Å, b = 6.2438 Å, c = 5.7903 Å (90.06º, 89.99º, 89.92º;
D2O, at ≈ 0.9 GPa and 80 K) [1582]. During the ordering of the hydrogen-bonding of ice VI, the a and b lattice constants contract, whereas there is a pronounced expansion in the c lattice constant [2687].
The three different hydrogen-bonding ordered structures, from [2687]
![the three different hydrogen-bonding ordered structures from [2687] the three different hydrogen-bonding ordered structures from [2687]](images/icexvr2.gif)
The two networks with ordered hydrogen-bonding can have one of three ordered hydrogen-bonding arrangements (A, B, C, see left) giving six different ordered structures; AA, BB, CC, AB, AC, and BC. With rotations of individual networks, there are 45 different crystalline structures possible [2687]. However, it appears that the found structure contains the highly polar C-type networks and is antiferroelectric.
The computational spectra of Raman, IR, and phonon density of states (PDOS) of ice XV have been described and analyzed [3770].
Ice-fifteen has triple points with ice-six and ice-two (estimated at 130 K, 0.8 GPa), and ice-six and ice-eight (estimated at 130 K, 1.5 GPa) [1582].
It appears (in 2017 [3023]) that another (mostly-) ordered form of (D2O) ice VI exists that is stable at lower temperatures (< 103 K) and different in structure to the form described above. Thus the earlier form of ice-fifteen (still called ice XV ) is stable between 103 K and 129 K, and the new form is called ice β-XV, now proposed as ice XIX [3987] has been demonstrated by neutron diffraction measurements. Originally it was not known which structure ice β-XV has out of the 45 different crystalline structures possible. More recently, this new form of ice XV has been questioned, with its kinetic features proposed to be related to glass transitions of deep glassy states [3453]. It has further been established that partially ordered states may exist in a mixture of ordered domains within disordered ice VI [3252]. However, the ![]() structure has been confirmed [3987] as one of two possible structures. The tetragonal lattice parameters at 70 K are a = 8.834 721(33) Å, c = 5.755 42(5) Å, volume = 449.224(4) Å3, with seven water molecules in the unit cell.
structure has been confirmed [3987] as one of two possible structures. The tetragonal lattice parameters at 70 K are a = 8.834 721(33) Å, c = 5.755 42(5) Å, volume = 449.224(4) Å3, with seven water molecules in the unit cell.
Ice XIX forms from ice VI on cooling to about 120 K at 1.8 GPa, with the accelerating dopant HCl. It also forms as a mixture with ice XV if the cooling is at 1.4 GPa, whereas ice XV is formed by itself on cooling at 0.85 GPa [4363]. It is denser than ice VI, and this stabilizes it under higher pressures. A transition from ice XIX to ice XV occurs upon heating and is the first order-to-order transition in the H-sublattice known for any water ice. Using neutron diffraction, ice XIX formed from HCl-doped ice VI is shown to form through a Pbcn-type distortion (at low temperatures and~1.6 GPa) which includes the tilting and squishing of the hexameric clusters [4290]. On warming Ice XIX at 0.1 MPa, it converts to Ice XV, and then to Ice VI [4363]. The ice XIX → ice XV transition at ambient pressure is the (so far) only order-to-order transition of ice [4447].
Interactive Jmol structures of ice VI and ice XV are given. [4290]
[Back to Top ![]() ]
]
a Two ice-nineteens have been proposed, see also [4057]. The superionic one is now called ice-twenty. [Back]
Home | Site Index | Phase Diagram | Ices, introduction | Ice-Ih | Ice-Ic | Ice-Isd | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | XIII | XIV | XVI| XVII | XVIII | Amorphous ice | LSBU | Top
This page was established in 2009 and last updated by Martin Chaplin on 29 April, 2022