
A commonly held delusion is that cars can be driven with just water as fuel.
Oh ye seekers after perpetual motion, how many vain chimeras have you pursued?
Go and take your place with the alchemists
Leonardo da Vinci, from his notebooks,, (1494)
if your theory is found to be against the second law of thermodynamics, I can give you no hope; there is nothing for it but to collapse in deepest humiliation.
Sir Arthur Stanley Eddington, The Nature of the Physical World (1927)
Alkaline fuel cell
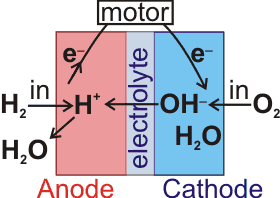
I have been approached many times by people who believe that water alone can be used in the net generation of power. However sincere these people are and however much they have invested, they are mistaken, and they have wasted their money. One of their (mistaken) ideas is to electrolyze water and then use the gases produced (H2, O2) to generate the power.
The hydrogen produced by electrolysis may be used as a fuel in a fuel cell (see right). However, the efficiency of the overall process (synthesis of H2 from H2O followed by oxidation of H2 to H2O) is always well below 100%. Thus the hydrogen produced can never be used to drive the electrolysis that produces it [2689]. This fact is governed by the unbreakable laws of thermodynamics but often seems to be ignored by people proposing cars that run on 'water' as a fuel or 'Brown's gas' (a mixture of H2 and O2 produced by electrolysis [1879]). Generating hydrogen by electrolysis is only (optimally) about 60% efficient, and the use of this hydrogen in a car is (optimally) also only about 60% efficient, so two-thirds of the energy required (0.6 ˣ 0.6 = 0.36) is wasted. The only time excess energy may apparently be produced (on a laboratory scale) is when the electrodes themselves react; an important factor (often ignored) that produces artifacts when using some stainless steel electrodes.
Water can be oxidized by singlet oxygen (1O2, the zero spin state of molecular oxygen where all the electrons are spin paired) to hydrogen peroxide,
1O2 + 2H2O ![]() 2H2O2
2H2O2
This reaction cannot be used as a fuel as the singlet oxygen is difficult and energetically expensive to produce. It is however used by the immune system to help the immunoglobulins destroy unwanted molecules.
Hidden ways of expecting to get energy out of 'burning' water, such as using an aqueous soup of biological or flammable material, cannot gain any energy from the water and are also fanciful (see, for example, 'Blinded by the Sun' by Stephen Poliakoff). However, the soot emission is significantly reduced from water-emulsified diesel fuel with a slight increase in combustion efficiency [3421].
An alternative source of energy in water electrolysis has been proposed [1875]. It involves 'cold fusion' (low energy nuclear reactions), a highly controversial theory developed from experiments involving electrolysis of heavy water using palladium (Pd) cathodes and reportedly producing greater heat than could be conventionally explained. This idea has received only limited acceptance with the main criticisms being a lack of a suitable theoretical basis and difficulties reproducing the results [3605]. History will decide [3965].
[Back to Top ![]() ]
]
Home | Site Index | Electrolysis | Electrical and magnetic effects on water | LSBU | Top
This page was established in 2012 and last updated by Martin Chaplin on 5 November, 2021