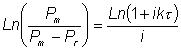|
|
Use of bead mills
Bead mills are available in various sizes and configurations from the Mickle shaker which has a maximum volume of about 40 ml to continuous process equipment capable of handling up to 200 Kg wet yeast or 20 Kg wet bacteria each hour. The bead mills that have been studied in most detail are the Dyno-Mill and the Netsch-Molinex agitator, both of which consist of a cylindrical vessel containing a motor-driven central shaft equipped with impellers of different types. Both can be operated continuously, being equipped with devices which retain the beads within the milling chamber. Glass Ballotini or stainless steel balls are used, the size range being selected for most effective release of the enzyme required. Thus 1 mm diameter beads are satisfactory for the rapid release of periplasmic enzymes from yeast but 0.25 mm diameter beads must be used, for a longer period, to release membrane-bound enzymes from bacteria. The kinetics of protein release from bead mills follows the relationship given by Equation 2.9 with respect to the time (t) that a particle spends in the mill. Unfortunately, however well designed these mills are, when continuously operated there will be a significant amount of backmixing which reduces the efficiency of the protein released with respect to the average residence time (t, see the discussion concerning backmixing in reactors in Chapter 5). This is more noticeable at low flow rates (high average residence times) and when the proportion of protein released is high. It may be counteracted by designing the bead mill to encourage plug flow characteristics. Under these circumstances the relationship can be shown to be where i represents the degree of backmixing (i.e., i = 0 under ideal plug flow conditions and i = 1 for ideal complete backmixing). Equation 2.11 reduces to give the simplified relationship of Equation 2.9 at low (near zero) values of i. In addition to bead size, the protein release rate constant (k) is a function of temperature, bead loading, impeller rotational speed and cell loading. Impeller speeds can be increased with advantage until bead breakage becomes significant but heat generation will also increase. At a constant impeller speed, the efficiency of the equipment declines with throughput as the degree of backmixing increases. There will be an optimum impeller tip speed at which the increases in disruption are balanced by increases in backmixing. In general, increased bead loading increases the rate of protein release but also increases the production of heat and the power consumption. Heat production is the major problem in the use of bead mills for enzyme release, particularly on a large (e.g., 20 litres) scale. Smaller vessels may be cooled adequately through cooling jackets around the bead chamber but larger mills require cooling through the agitator shaft and impellers. However, if cooling is effective there is little damage to the enzymes released.
This page was established in 2004 and last updated by Martin
Chaplin |