|
|
Enzymic reactions in biphasic liquid systems
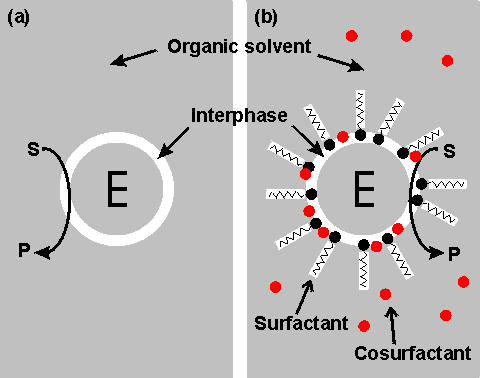
Some polymerising reactions, for example the polymerisation of phenols catalysed by peroxidase, will produce a higher molecular weight product when carried out in a solution more able to dissolve the products (i.e., oligomers) initially formed. Under normal physiological conditions, hydrolytic enzymes catalyse the degradation of polymers; i.e., hydrolases are transferases normally transferring a moiety to the acceptor, water. Water is normally present in a vast molar excess over other potential acceptor molecules so no reaction occurs other than hydrolysis. Also, the normal 'concentration' of water (about 55.5 M) is much greater than its typical Km (about 50 mM) and the rate of hydrolysis will not be affected as the reaction proceeds. By greatly reducing the water activity in these systems they can be used to transfer to other acceptors. Examples of this can be found in the transesterification reactions of esterases and lipases, described more fully later, and the (undesirable) formation of isomaltose from glucose catalysed by glucoamylase. Restriction of the enzyme to the aqueous phase effectively immobilises the enzyme and allows its straightforward separation, using phase separators developed for the established chemical process industry, from product-containing organic phase. The main asset of these systems, however, is their ability to shift the thermodynamic equilibria of the reactions. As was pointed out in Chapter 1, enzymes do not change the equilibrium constants (Keq) of reactions. Although changes in the physical conditions (i.e., temperature, pH and pressure) do affect the Keq of a reaction, usually this effect is relatively slight over the physical range allowed by stability of the biocatalysts. Use of a biphasic aqueous-organic system, however, may result in substantial changes in the practically useful 'apparent' Keq. The use of enzymes within organic solvents normally results in a two phase system as all water-soluble enzymes possess a significant amount of strongly bound water, even when in an apparently dry state. This is shown schematically in Figure 7.1.
Figure 7.1. Schematic diagram showing two configurations for an enzyme within an organic solvent. (a) almost-anhydrous enzyme suspended in the organic solvent. The enzyme (E) is surrounded by a thin interphase consisting of water or water plus immobilisation support. (b) enzyme dissolved in a reversed micellar medium. The micelles are formed by the surfactant molecules with assistance from the cosurfactant (if present). The surfactant (e.g., cetyltrimethylammonium bromide (CTAB), bis(2-ethylhexyl) sodium sulphosuccinate (AOT), phosphatidylcholine, tetraethyleneglycoldodecylether) is only found at the interphase boundary, whereas the water-immiscible cosurfactant (e.g., butanol, hexanol, octanol), added to vary the properties of this interphase, is generally less polar and more soluble in the organic continuous phase. Both preparations (a) and (b) give optically transparent solutions.
This page was established in 2004 and last updated by Martin
Chaplin |