|
|
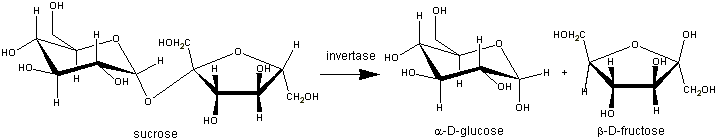
Enzymes in the sucrose industryThe sucrose industry is a comparatively minor user of enzymes but provides few historically significant and instructive examples of enzyme technology The hydrolysis ('inversion') of sucrose, completely or partially, to glucose and fructose provides sweet syrups that are more stable (i.e., less likely crystallise) than pure sucrose syrups. The most familiar 'Golden Syrup' produced by acid hydrolysis of one of the less pure streams from the cane sugar refinery but other types of syrup are produced using yeast (Saccharomyces cerevisiae) invertase. Although this enzyme is unusual in that it suffers from substrate inhibition at high sucrose levels ( > 20% (w/w)), this does not prevent its commercial use at even higher concentrations: Traditionally, invertase was produced on site by autolysing yeast cells. The autolysate was added to the syrup (70% sucrose (w/w)) to be inverted together with small amounts of xylene to prevent microbial growth. Inversion was complete in 48 - 72 h at 50°C and pH 4.5. The enzyme and xylene were removed during the subsequent refining and evaporation. Partially inverted syrups were (and still are) produced by blending totally inverted syrups with sucrose syrups. Now, commercially produced invertase concentrates are employed. The production of hydrolysates of a low molecular weight compound in essentially pure solution seems an obvious opportunity for the use of an immobilised enzyme, yet this is not done on a significant scale, probably because of the extreme simplicity of using the enzyme in solution and the basic conservatism of the sugar industry. Invertase finds another use in the production of confectionery with liquid or soft centres. These centres are formulated using crystalline sucrose and tiny (about 100 U kg−1, 0.3 ppm (w/w)) amounts of invertase. At this level of enzyme, inversion of sucrose is very slow so the centre remains solid long enough for enrobing with chocolate to be completed. Then, over a period of days or weeks, sucrose hydrolysis occurs and the increase in solubility causes the centres to become soft or liquid, depending on the water content of the centre preparation. Other enzymes are used as aids to sugar production and refining by removing materials which inhibit crystallisation or cause high viscosity. In some parts of the world, sugar cane contains significant amounts of starch, which becomes viscous, thus slowing filtration processes and making the solution hazy when the sucrose is dissolved. This problem can be overcome by using the most thermostable a-amylases (e.g., Termamyl at about 5 U kg−1) which are entirely compatible with the high temperatures and pH values that prevail during the initial vacuum evaporation stage of sugar production. Other problems involving dextran and raffinose required the development of new industrial enzymes. A dextran is produced by the action of dextransucrase (EC 2.4.1.5) from Leuconostoc mesenteroides on sucrose and found as a slime on damaged cane and beet tissue, especially when processing has been delayed in hot and humid climates. Raffinose, which consists of sucrose with a-galactose attached through its C-1 atom to the 6 position on the glucose residue, is produced at low temperatures in sugar beet. Both dextran and raffinose have the sucrose molecule as part of their structure and both inhibit sucrose crystal growth. This produces plate-like or needle-like crystals which are not readily harvested by equipment designed for the approximately cubic crystals otherwise obtained. Dextran can produce extreme viscosity it process streams and even bring plant to a stop. Extreme dextran problems arc frequently solved by the use of fungal dextranases produced from Penicillium species. These are used (e.g., 10 U kg−1 raw juice, 55°C, pH 5.5, 1 h) only in times of crisis as they are not sufficiently resistant to thermal denaturation for long-term use and are inactive at high sucrose concentrations. Because only small quantities are produced for use, this enzyme is relatively expensive. An enzyme sufficiently stable for prophylactic use would be required in order to benefit from economies of scale. Raffinose may be hydrolysed to galactose and sucrose by a fungal raffinase (see Chapter 5).
This page was established in 2004 and last updated by Martin
Chaplin |