|
|
Kinetics of immobilised enzymesThe kinetic behaviour of a bound enzyme can differ significantly from that of the same enzyme in free solution. The properties of an enzyme can be modified by suitable choice of the immobilisation protocol, whereas the same method may have appreciably different effects on different enzymes. These changes may be due to conformational alterations within the enzyme due to the immobilisation procedure, or the presence and nature of the immobilisation support. Immobilisation can greatly effect the stability of an enzyme. If the immobilisation process introduces any strain into the enzyme, this is likely to encourage the inactivation of the enzymes under denaturing conditions (e.g., higher temperatures or extremes of pH). However where there is an unstrained multipoint binding between the enzyme and the support, substantial stabilisation may occur (Figure 3.5). This is primarily due to the physical prevention of the large conformational changes within the protein structure which generally precede its inactivation. Many successful covalent immobilisation processes involve an initial freely-reversible stage, where the covalent links are allowed to form, break and re-form until an unstrained covalently-linked structure is created, in order to stabilise the resultant immobilised enzyme. Additional stabilisation is derived by preventing the enzyme molecules from interacting with each other, and the protection that immobilisation affords towards proteolytic and microbiological attack. This latter effect is due to a combination of diffusional difficulties and the camouflage to enzymic attack produced by the structural alterations. In order to achieve maximum stabilisation of the enzymes, the surfaces of the enzyme and support should be complementary with the formation of many unstrained covalent or non-covalent interactions. Often, however, this factor must be balanced against others, such as the cost of the process, the need for a specific support material, and ensuring that the substrates are not sterically hindered from diffusing to the active site of the immobilised enzyme in order to react at a sufficient rate.
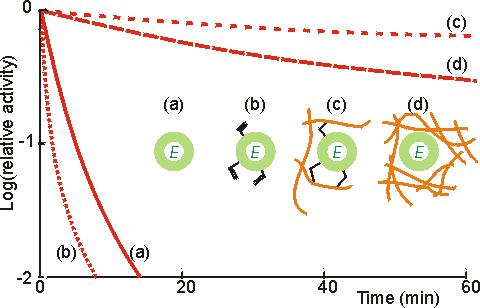
Figure 3.5. Illustration of the use of multipoint interactions for the stabilisation of enzymes (Martinek et al, 1977a,b). (a) ——— activity of free underivatised chymotrypsin. (b) ········· activity of chymotrypsin derivatised with acryloyl chloride. (c) -------- activity of acryloyl chymotrypsin copolymerised within a polymethacrylate gel. Up to 12 residues are covalently bound per enzyme molecule. Lower derivatisation leads to lower stabilisation. (d) ----- activity of chymotrypsin non-covalently entrapped within a polymethacrylate gel. The degree of stabilisation is determined by strength of the gel, and hence the number of non-covalent interactions. All reactions were performed at 60°C using low molecular weight artificial substrates. The immobilised chymotrypsin preparations showed stabilisation of up to 100,000 fold, most of which is due to their multipoint nature although the consequent prevention of autolytic loss of enzyme activity must be a significant contributory factor. The kinetic constants (e.g., Km, Vmax) of enzymes may be altered by the process of immobilisation due to internal structural changes and restricted access to the active site. Thus, the intrinsic specificity (k./Km) of such enzymes may well be changed relative to the soluble enzyme. An example of this involves trypsin where the freely soluble enzyme hydrolyses fifteen peptide bonds in the protein pepsinogen but the immobilised enzyme hydrolyses only ten. The apparent value of these kinetic parameters, when determined experimentally, may differ from the intrinsic values. This may be due to changes in the properties of the solution in the immediate vicinity of the immobilised enzyme, or the effects of molecular diffusion within the local environment (Figure 3.6). The relationship between these parameters is shown below. Intrinsic parameters of the soluble
enzyme
Figure 3.6. A schematic cross-section of an immobilised enzyme particle (a) shows the macroenvironment and microenvironment. triangular dots represent the enzyme molecules. The microenvironment consists of the internal solution plus that part of the surrounding solution which is influenced by the surface characteristics of the immobilised enzyme. Partitioning of substances will occur between these two environments. Substrate molecules (S) must diffuse through the surrounding layer (external transport) in order to reach the catalytic surface and be converted to product (P). In order for all the enzyme to be utilised, substrate must also diffuse within the pores in the surface of the immobilised enzyme particle (internal transport). The porosity (e) of the particle is the ratio of the volume of solution contained within the particle to the total volume of the particle. The tortuosity (t) is the average ratio of the path length, via the pores, between any points within the particle to their absolute distance apart. The tortuosity, which is always greater than or equal to unity, clearly depends on the pore geometry. The diagram exaggerates dimensions for the purpose of clarity. Typically, the diameter of enzyme molecules (2 - 10 nm) are 1 - 2 decades smaller than the pore diameters which are 2 - 4 decades smaller than the particle diameters (10 - 2000 mm); the microenvironment consisting of a diffusion layer (≈ 10 mm thick) and a thinner partition layer (≈ 20 nm thick). (b) The concentration of the substrate at the surface of the particles (radius R) is [SR] whereas the internal concentration at any smaller radius (r) is the lower value represented by [Sr].
This page was established in 2004 and last updated by Martin
Chaplin |
![Porous bead diameter R, substrate concentration at radius r is [Sr]](images/fig3_6.gif)