|
|
FiltrationFiltration separates simply on the basis of particle size. Its efficiency is limited by the shape and compressibility of the particles, the viscosity of the liquid phase and the maximum allowable pressures. Large-scale simple filtration employs filter cloths and filter aids in a plate and frame press configuration, in rotary vacuum filters or centrifugal filters (Figure 2.3). The volumetric throughput of a filter is proportional to the pressure (P) and filter area (AF) and inversely proportional to the filter cake thickness (DF) and the dynamic viscosity where k is a proportionality constant dependent on the size and nature of the particles. For very small particles k depends on the fourth power of their diameter. Filtration of particles that are easily compressed leads to filter blockage and the failure of Equation 2.3 to describe the system. Under these circumstances a filter aid, such as celite, is mixed with the feedstock to improve the mechanical stability of the filter cake. Filter aids are generally used only where the liquid phase is required as they cause substantial problems in the recovery of solids. They also may cause loss of enzyme activity from the solution due to physical hold-up in the filter cake. It is often difficult for a process development manager to decide whether to attempt to recover enzyme trapped in this way. Problems associated with the build-up of the filter cake may also be avoided by high tangential flow of the feedstock across the surface of the filter, a process known as crossflow microfiltration (Figure 2.4). This method dispenses with filter aids and uses special symmetric microporous membrane assemblies capable of retaining particles down to 0.1 - 1 mm diameter (compare Bacillus diameter of about 2 mm).
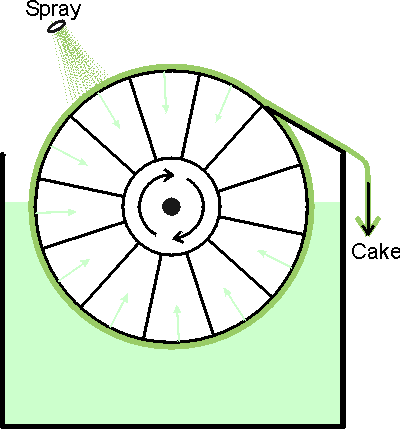
Figure 2.3. The basic design of the rotary vacuum filter. The suspension is sucked through a filter cloth on a rotating drum. This produces a filter cake which is removed with a blade. The filter cake may be rinsed during its rotation. These filters are generally rather messy and difficult to contain making them generally unsuitable for use in the production of toxic or recombinant DNA products. There have been recent developments that improve their suitability, however, such as the Disposable Rotary Drum Filter. A simple and familiar filtration apparatus is the perforate bowl centrifuge or basket centrifuge, in effect a spin drier. Cell debris is collected on a cloth with, or without, filter aid and can be skimmed off when necessary using a suitable blade. Such centrifugal filters have a large radius and effective liquid depth, allowing high volumes. However, safety decrees that the angular velocity must be low and so only large particles (e.g., plant material) can be removed satisfactorily.
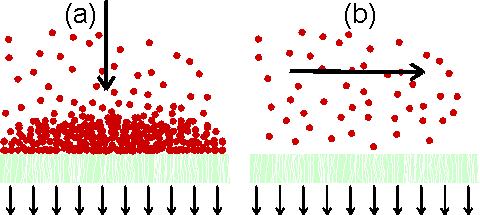
Figure 2.4. Principles of (a) dead-end filtration and (b) cross-flow filtration. In dead-end filtration the flow causes the build-up of the filter cake, which may prevent efficient operation. This is avoided in cross-flow filtration where the flow sweeps the membrane surface clean.
This page was established in 2004 and last updated by Martin
Chaplin |