|
|
Coenzyme-regenerating systemsMany oxidoreductases and all ligases utilise coenzymes (e.g., NAD+, NADP+, NADH, NADPH, ATP), which must be regenerated as each product molecule is formed. Although these represent many of the most useful biological catalysts, their application is presently severely limited by the high cost of the coenzymes and difficulties with their regeneration. These two problems may both be overcome at the same time if the coenzyme is immobilised, together with the enzyme, and regenerated in situ. A simple way of immobilising/regenerating coenzymes would be to use whole-cell systems and these are, of course, in widespread use. However as outlined earlier, these are of generally lower efficiency and flexibility than immobilised-enzyme systems. Membrane reactors (see Chapter 5) may be used to immobilise the coenzymes but the pore size must be smaller than the coenzyme diameter, which is extremely restrictive. Coenzymes usually must be derivatised for adequate immobilisation and regeneration. When successfully applied, this process activates the coenzymes for attachment to the immobilisation support but does not interfere with its biological function. The most widely applied synthetic routes involve the alkylation of the exocyclic N6-amino nitrogen of the adenine moiety present in the coenzymes NAD+, NADP+, NADH, NADPH, ATP and coenzyme A. In some applications, such as those using membrane reactors it is only necessary that the coenzyme has sufficient size to be retained within the system. High molecular weight water-soluble derivatives are most useful as they cause less diffusional resistance than insoluble coenzyme matrices. Dextrans, polyethyleneimine and polyethylene glycols are widely used. Relatively low levels of coenzyme attachment are generally sought in order to allow greater freedom of movement and avoid possible inhibitory effects. The kinetic properties of the derived coenzymes vary, depending upon the system, but generally the Michaelis constants are higher and the maximum velocities are lower than with the native coenzymes. Coenzymes immobilised to insoluble supports presently have somewhat less favourable kinetics even when co-immobilised close to the active site of their utilising enzymes. This situation is expected to improve as more information on the protein conformation surrounding the enzymes' active sites becomes available and immobilisation methods become more sophisticated. However, the cost of such derivatives is always likely to remain high and they will only be economically viable for the production of very high value products. There are several systems available for the regeneration of the derivatised coenzymes by chemical, electrochemical or enzymic means. Enzymic regeneration is advantageous because of its high specificity but electrochemical procedures for regenerating the oxidoreductase dinucleotides are proving competitive. To be useful in regenerating coenzymes, enzymic processes must utilise cheap substrates and readily available enzymes and give non-interfering and easily separated products. Formate dehydrogenase and acetate kinase present useful examples of their use, although the presently available commercial enzyme preparations are of low activity:
This page was established in 2004 and last updated by Martin
Chaplin |
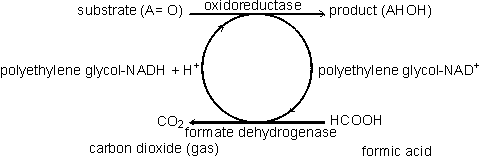 [8.7]
[8.7]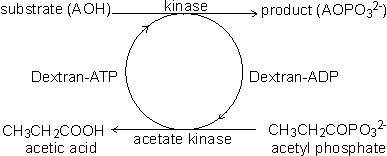 [8.8]
[8.8]